
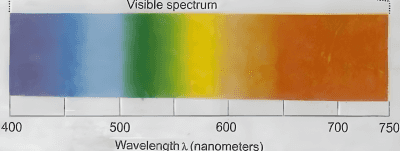
Which of the following statements are correct, if the threshold frequency of caesium is $5.16 \times$ $10^{14} \mathrm{~Hz}$ ?
A. When Cs is placed inside a vacuum chamber with an ammeter connected to it and yellow light is focused on Cs , the ammeter shows the presence of current.
B. When the brightness of the yellow light is dimmed, the value of the current in the ammeter is reduced.
C. When a red light is used instead of the yellow light, the current produced is higher with respect to the yellow light.
D. When a blue light is used, the ammeter shows the formation of current.
E. When a white light is used. the ammeter shows formation of current.
Choose the correct answer from the options given below:

The group 14 elements $A$ and $B$ have the first ionisation enthalpy values of 708 and $715 \mathrm{~kJ} \mathrm{~mol}^{-1}$ respectively. The above values are lowest among their group members. The nature of their ions $\mathrm{A}^{2+}$ and $\mathrm{B}^{4+}$ respectively is

The reactions which cannot be applied to prepare an alkene by elimination, are

Choose the correct answer from the options given below:

When a salt is treated with sodium hydroxide solution it gives gas X . On passing gas X through reagent Y a brown coloured precipitate is formed. X and Y respectively, are