Chemistry
K1 = 4.2 x 10–7 and K2 = 4.8 x 10–11
Select the correct statement for a saturated 0.034 M solution of the carbonic acid.
(i) H3PO4 + H2O $$\to$$ H3O+ + $$H_2PO_4^−$$
(ii) $$H_2PO_4^−$$ + H2O $$\to$$ $$HPO_4^{2−}$$ + H3O+
(iii) $$H_2PO_4^−$$ + OH- $$\to$$H3PO4 + O2-
In which of the above does $$H_2PO_4^−$$ act as an acid?
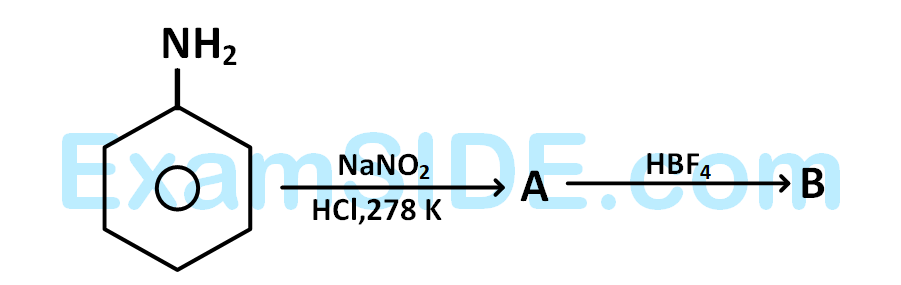
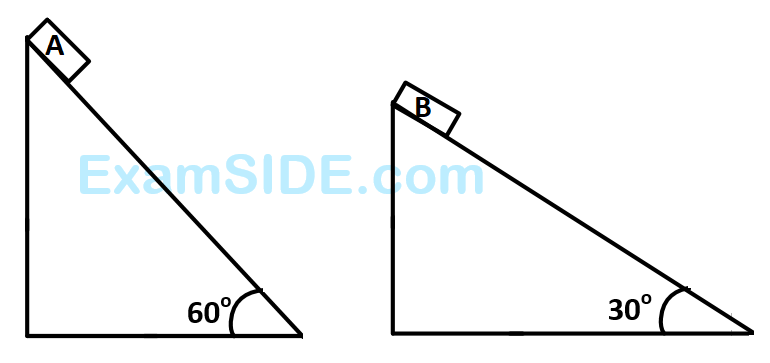
the compounds $$'A'$$ and $$'B'$$ respectively are
$$\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,$$ $${C_6}{H_5}C{H_2}CH\left( {OH} \right)CH{\left( {C{H_3}} \right)_2}\buildrel {conc.{H_2}S{O_4}} \over \longrightarrow \,?$$
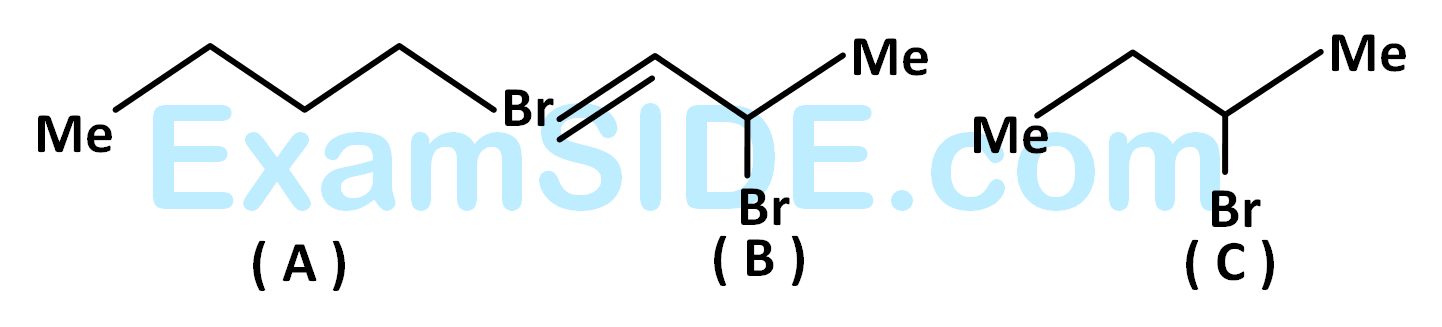
The correct order of $${S_N}1$$ reactive is
(en = ethylenediamine)
Consider the reaction :
Cl2(aq) + H2S(aq) → S(s) + 2H+ (aq) + 2Cl– (aq)
The rate equation for this reaction is rate = k [Cl2] [H2S]
Which of these mechanisms is/are consistent with this rate equation?
(A) Cl2 + H2S $$\to$$ H+ + Cl– + Cl+ + HS– (slow)
Cl+ + HS– $$\to$$ H+ + Cl– + S (fast)
(B) H2S $$ \Leftrightarrow $$ H+ + HS– (fast equilibrium)
Cl2 + HS– $$\to$$ 2Cl– + H+ + S (slow)
$${2 \over 3}A{l_2}{O_3}$$ $$\to$$ $${4 \over 3}Al + {O_2}$$, $${\Delta _r}G$$ = + 966 kJ mol–1
The potential difference needed for electrolytic reduction of Al2O3 at 500oC is at least :
(c = 3 x 108 ms–1 and NA = 6.02 x 1023 mol–1)
Mathematics
$$\mathop {\lim }\limits_{x \to \infty } {{f(3x)} \over {f(x)}} = 1$$. Then $$\mathop {\lim }\limits_{x \to \infty } {{f(2x)} \over {f(x)}} = $$
If $$f$$has a local minimum at $$x=-1$$, then a possible value of $$k$$ is
Statement - 1: The probability that the chosen numbers when arranged in some order will form an AP is $${1 \over {85}}.$$
Statement - 2: If the four chosen numbers form an AP, then the set of all possible values of common difference is $$\left( { \pm 1, \pm 2, \pm 3, \pm 4, \pm 5} \right).$$
$$\cos x\,dy = y\left( {\sin x - y} \right)dx,\,\,0 < x <{\pi \over 2}$$ is :
The system has :
where $$I$$ is $$2 \times 2$$ identity matrix. Define
$$Tr$$$$(A)=$$ sum of diagonal elements of $$A$$ and $$\left| A \right| = $$ determinant of matrix $$A$$.
Statement- 1: $$Tr$$$$(A)=0$$.
Statement- 2: $$\left| A \right| = 1$$ .
Statement - 1 : $$f\left( c \right) = {1 \over 3},$$ for some $$c \in R$$.
Statement - 2 : $$0 < f\left( x \right) \le {1 \over {2\sqrt 2 }},$$ for all $$x \in R$$
Then $$tan\,2\alpha $$ =
$R=\{(x, y) \mid x, y$ are real numbers and $x=w y$ for some rational number $w\}$;
$S=\left\{\left(\frac{m}{n}, \frac{p}{q}\right) \mid m, n, p\right.$ and $q$ are integers such that $n, q \neq 0$ and $q m=p m\}$. Then
Physics
The speed of daughter nuclei is
The binding energy per nucleon for the parent nucleus is $${E_1}$$ and that for the daughter nuclei is $${E_2}.$$ Then
Statement - $$2$$ : Photoelectrons are emitted with speeds ranging from zero to a maximum value because of the range of frequencies present in the incident light.
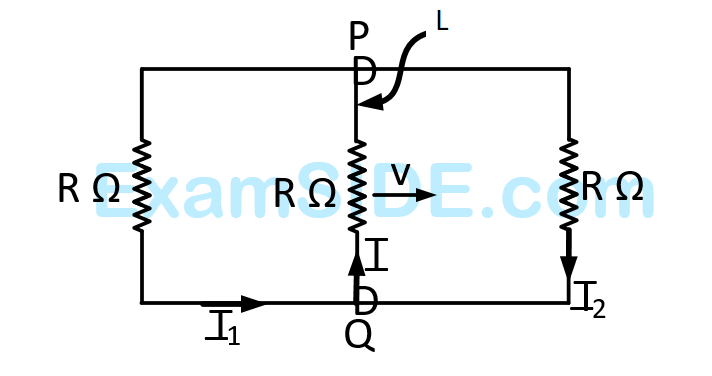
The speed of light in the medium is
The initial shape of the wavefront of the beam is
As the beam enters the medium, it will
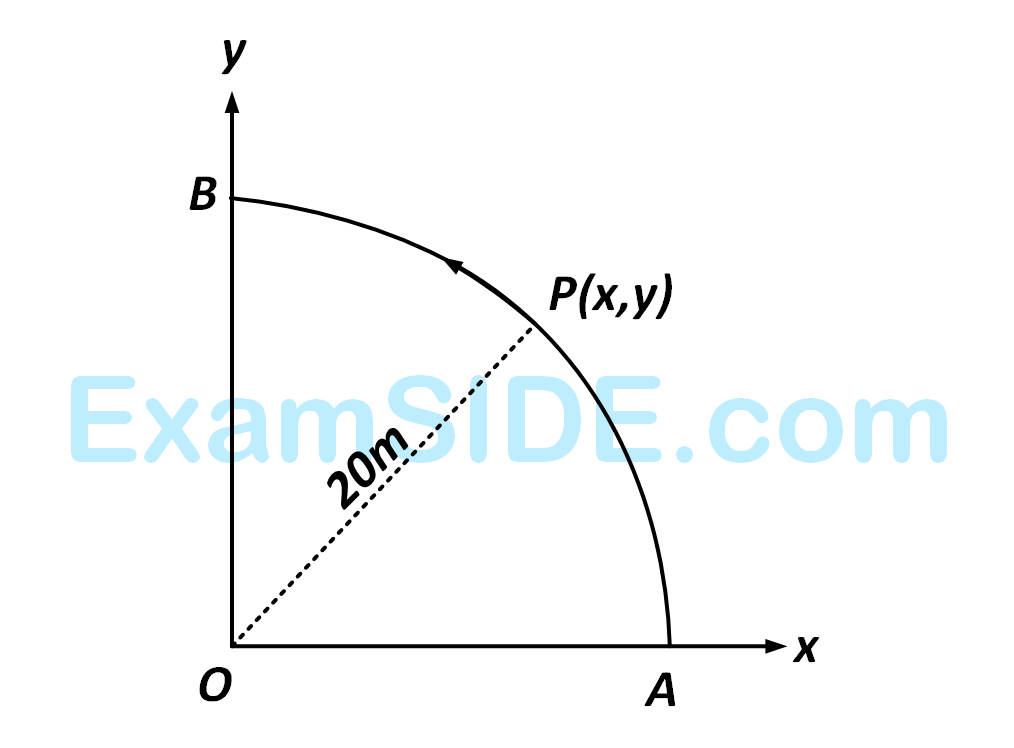
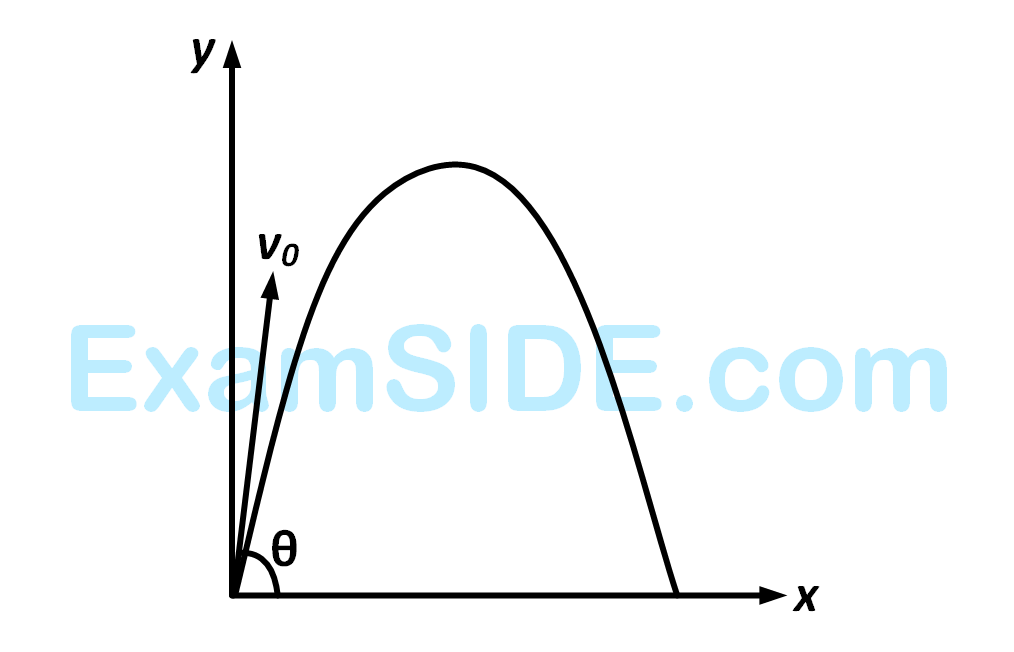
where $$\widehat i,\widehat j$$ and $$\widehat k$$ are unit vectors along $$x,y$$ and $$z$$-axis respectively.

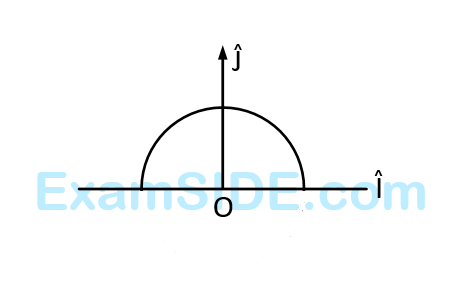
The tension in the string is
Statement - 2 : Principle of conservation of momentum holds true for all kinds of collisions.