
Given below are two statements :
Statement I : Mohr's salt is composed of only three types of ions-ferrous, ammonium and sulfate.
Statement II : If the molar conductance at infinite dilution for ferrous, ammonium and sulfate ions are $x_1, x_2$ and $x_3 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}$, respectively then the molar conductance for Mohr's salt solution at infinite dilution would be given by $x_1+x_2+2 x_3$
In the light of the given statements, choose the correct answer from the options given below :

Given below are two statements:
Statement I: D-(+)-glucose + D-(+) fructose $\xrightarrow{-\mathrm{H}_2 \mathrm{O}}$ Sucrose
$$\text { sucrose } \xrightarrow{\text { hydrolysis }} \mathrm{D}-(+) \text { glucose }+\mathrm{D}-(+) \text { fructose }$$
Statement II: Invert sugar is formed during sucrose hydrolysis
In the light of the above statements, choose the correct answer from the options given below

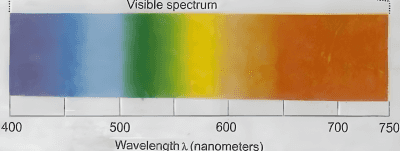
Which of the following statements are correct, if the threshold frequency of caesium is $5.16 \times$ $10^{14} \mathrm{~Hz}$ ?
A. When Cs is placed inside a vacuum chamber with an ammeter connected to it and yellow light is focused on Cs , the ammeter shows the presence of current.
B. When the brightness of the yellow light is dimmed, the value of the current in the ammeter is reduced.
C. When a red light is used instead of the yellow light, the current produced is higher with respect to the yellow light.
D. When a blue light is used, the ammeter shows the formation of current.
E. When a white light is used. the ammeter shows formation of current.
Choose the correct answer from the options given below:

The group 14 elements $A$ and $B$ have the first ionisation enthalpy values of 708 and $715 \mathrm{~kJ} \mathrm{~mol}^{-1}$ respectively. The above values are lowest among their group members. The nature of their ions $\mathrm{A}^{2+}$ and $\mathrm{B}^{4+}$ respectively is