
Given below are two statements :
Statement I : Dimethyl ether is completely soluble in water. However, diethyl ether is soluble in water to a very small extent.
Statement II : Sodium metal can be used to dry diethyl ether and not ethyl alcohol.
In the light of given statements. choose the correct answer from the options given below

Given below are two statements :
Statement I : Mohr's salt is composed of only three types of ions-ferrous, ammonium and sulfate.
Statement II : If the molar conductance at infinite dilution for ferrous, ammonium and sulfate ions are $x_1, x_2$ and $x_3 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}$, respectively then the molar conductance for Mohr's salt solution at infinite dilution would be given by $x_1+x_2+2 x_3$
In the light of the given statements, choose the correct answer from the options given below :

Given below are two statements:
Statement I: D-(+)-glucose + D-(+) fructose $\xrightarrow{-\mathrm{H}_2 \mathrm{O}}$ Sucrose
$$\text { sucrose } \xrightarrow{\text { hydrolysis }} \mathrm{D}-(+) \text { glucose }+\mathrm{D}-(+) \text { fructose }$$
Statement II: Invert sugar is formed during sucrose hydrolysis
In the light of the above statements, choose the correct answer from the options given below

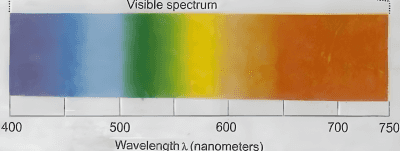
Which of the following statements are correct, if the threshold frequency of caesium is $5.16 \times$ $10^{14} \mathrm{~Hz}$ ?
A. When Cs is placed inside a vacuum chamber with an ammeter connected to it and yellow light is focused on Cs , the ammeter shows the presence of current.
B. When the brightness of the yellow light is dimmed, the value of the current in the ammeter is reduced.
C. When a red light is used instead of the yellow light, the current produced is higher with respect to the yellow light.
D. When a blue light is used, the ammeter shows the formation of current.
E. When a white light is used. the ammeter shows formation of current.
Choose the correct answer from the options given below: