Chemistry
(Ksp for BaCO3 = 5.1 $$\times$$ 10−9 )
($$\Delta _fG^oH^+_{(aq)}$$ = 0)
H2O(l) $$\to$$ H+(aq) + OH-(aq); $$\Delta H$$ = 57.32 kJ
H2(g) + $${1 \over 2} O_2(g) \to$$ H2O(l); $$\Delta H$$ = -286.20 kJ
The value of enthalpy of formation of OH− ion at 25oC is :
2PhCHO $$\buildrel {\mathop {:OH}\limits^{\left( - \right)} } \over \longrightarrow $$ PhCH2OH + $$PhC\mathop O\limits^{..} $$2(-)
the slowest step is :
CH3OH(l) + 3/2O2 $$\to$$ CO2 (g) + 2H2O (l)
At 298K standard Gibb’s energies of formation for CH3OH(l), H2O(l) and CO2 (g) are -166.2, -237.2 and -394.4 kJ mol−1 respectively. If standard enthalpy of combustion of methanol is -726 kJ mol−1, efficiency of the fuel cell will be
The value of standard electrode potential for the change,
Fe3+ (aq) + e- $$\to$$ Fe2+ (aq) will be
Mathematics
Statement - 2 : The sum of first n natural numbers is $${{n\left( {n + 1} \right)} \over 2}$$ and the sum of squares of first n natural numbers is $${{n\left( {n + 1} \right)\left( {2n + 1} \right)} \over 6}$$
Statement - 1 : The set $$\left\{ {x:f\left( x \right) = {f^{ - 1}}\left( x \right)} \right\} = \left\{ {0, - 1} \right\}$$.
Statement - 2 : $$f$$ is a bijection.
$$\left| {\matrix{
a & {a + 1} & {a - 1} \cr
{ - b} & {b + 1} & {b - 1} \cr
c & {c - 1} & {c + 1} \cr
} } \right| + \left| {\matrix{
{a + 1} & {b + 1} & {c - 1} \cr
{a - 1} & {b - 1} & {c + 1} \cr
{{{\left( { - 1} \right)}^{n + 2}}a} & {{{\left( { - 1} \right)}^{n + 1}}b} & {{{\left( { - 1} \right)}^n}c} \cr
} } \right| = 0$$
then the value of $$n$$ :
real root of $$P'\,\left( x \right) = 0.$$ If $$P\left( { - 1} \right) < P\left( 1 \right),$$ then in the interval $$\left[ { - 1,1} \right]:$$
Statement-1: gof is differentiable at $$x=0$$ and its derivative is continuous at that point.
Statement-2: gof is twice differentiable at $$x=0$$.
A: $$\cos \alpha + \cos \beta + \cos \gamma = 0$$
B: $$\sin \alpha + \sin \beta + \sin \gamma = 0$$
If $$\cos \left( {\beta - \gamma } \right) + \cos \left( {\gamma - \alpha } \right) + \cos \left( {\alpha - \beta } \right) = - {3 \over 2},$$ then:
Physics


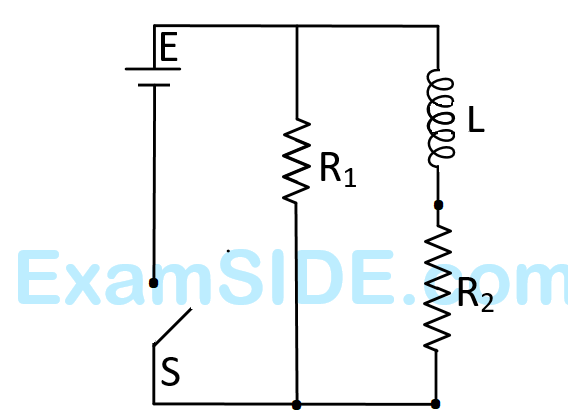
Output is

The current $$(I)$$ in the resistor $$(R)$$ can be shown by :
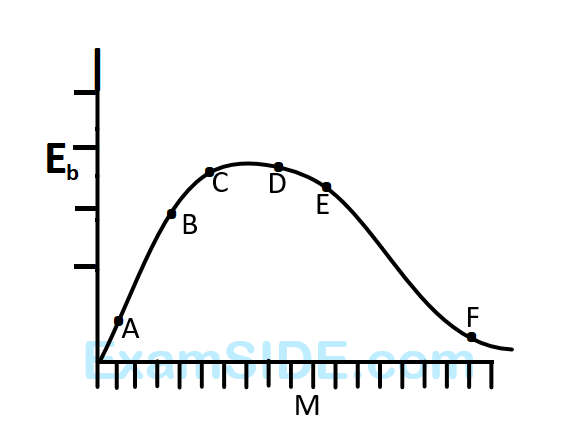
The above is a plot of binding energy per nucleon $${E_b},$$ against the nuclear mass $$M;A,B,C,D,E,F$$ correspond to different nuclei. Consider four reactions :
$$\eqalign{
& \left( i \right)\,\,\,\,\,\,\,\,\,\,A + B \to C + \varepsilon \,\,\,\,\,\,\,\,\,\,\left( {ii} \right)\,\,\,\,\,\,\,\,\,\,C \to A + B + \varepsilon \,\,\,\,\,\,\,\,\,\, \cr
& \left( {iii} \right)\,\,\,\,\,\,D + E \to F + \varepsilon \,\,\,\,\,\,\,\,\,\,\left( {iv} \right)\,\,\,\,\,\,\,\,\,F \to D + E + \varepsilon ,\,\,\,\,\,\,\,\,\,\, \cr} $$
where $$\varepsilon $$ is the energy released? In which reactions is $$\varepsilon $$ positive?

The incident angle $$\theta $$ for which the light ray grazes along the wall of the rod is :
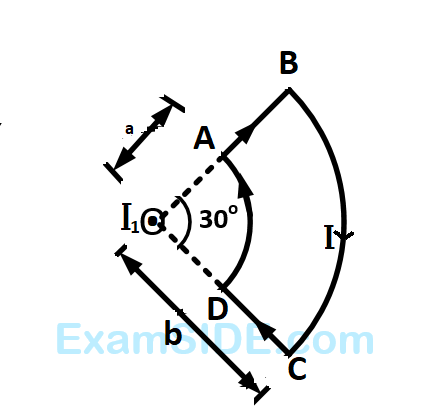
Due to the presence of the current $${I_1}$$ at the origin:

The magnitude of the magnetic field $$(B)$$ due to the loop $$ABCD$$ at the origin $$(O)$$ is :
Then the velocity as a function of time and the height as a function of time will be :
Statement-1 : For a charged particle moving from point $$P$$ to point $$Q$$, the net work done by an electrostatic field on the particle is independent of the path connecting point $$P$$ to point $$Q.$$
Statement-2 : The net work done by a conservative force on an object moving along a closed loop is zero.
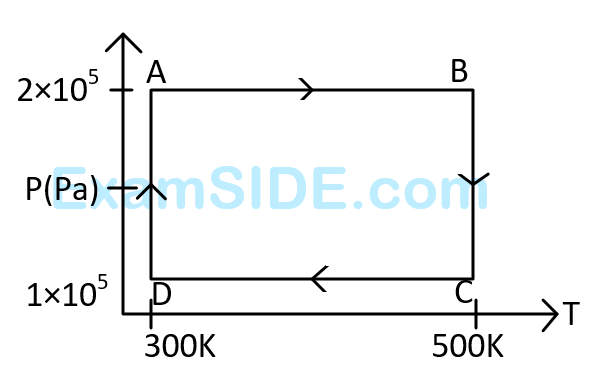
The net work done on the gas in the cycle $$ABCDA$$ is:
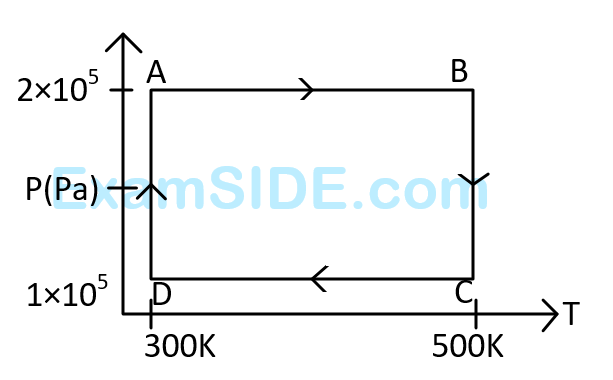
The work done on the gas in taking it from $$D$$ to $$A$$ is :

Assuming the gas to be ideal the work done on the gas in taking it from $$A$$ to $$B$$ is :
Statement - 2: $$R = {R_0}\left( {1 + \alpha \,\Delta t} \right)$$ is valid only when the change in the temperature $$\Delta T$$ is small and $$\Delta T = \left( {R - {R_0}} \right) < < {R_0}.$$