JEE Main 2020 (Online) 7th January Morning Slot
Paper was held on
Tue, Jan 7, 2020 3:30 AM
Chemistry
1
Chlorine reacts with hot and concentrated NaOH and produces compounds (X) and (Y). Compound
(X) gives white precipitate with silver nitrate solution. The average bond order between CI and O
atoms in (Y) is.
2
For the reaction :
A($$l$$) $$ \to $$ 2B(g)
$$\Delta U = 2.1\,kcal,\,\Delta S = 20\,cal\,{K^{ - 1}}$$ at 300 K
Hence $$\Delta $$G in kcal is :
A($$l$$) $$ \to $$ 2B(g)
$$\Delta U = 2.1\,kcal,\,\Delta S = 20\,cal\,{K^{ - 1}}$$ at 300 K
Hence $$\Delta $$G in kcal is :
3
During the nuclear explosion, one of the products is 90Sr with half life of 6.93 years. If 1 $$\mu $$ g of 90Sr was absorbed in the bones of newly born baby in placed of Ca, how much time, in years, is required to reduce much time, in year, is required to reduce it by 90% if it not lost metabolically.
4
Two solutions, A and B, each of 100L was made by dissolving 4g of NaOH and 9.8 g of H2SO4 in water, respectively. The pH of the resultant solution obtained from mixing 40L of solution A and 10L of solution B is :
5
The number of orbitals associated with quantum number n = 5, ms = +$${1 \over 2}$$ is :
6
What is the product of following reaction ?


7
Given that the standard potentials (Eo) of Cu2+/Cu and Cu+/Cu are 0.34 V and 0.522 V respectively, the Eo of Cu2+/Cu+ :
8
Consider the following reaction :

The product 'X' is used :

The product 'X' is used :
9
Consider the following reaction :
$${\left( {C{H_3}} \right)_3}CCH\left( {OH} \right)C{H_3}\buildrel {conc.\,\,\,{H_2}S{O_4}} \over \longrightarrow $$
$${\left( {C{H_3}} \right)_2}CHCH\left( {Br} \right)C{H_3}\buildrel {alc.\,\,KOH} \over \longrightarrow $$
$${\left( {C{H_3}} \right)_2}{\rm{ }}CHCH\left( {Br} \right)C{H_3}\buildrel {{{(C{H_3})}_3}{O^\Theta }{K^ \oplus }} \over \longrightarrow $$
$${\left( {C{H_3}} \right)_2}\mathop C\limits_{\mathop |\limits_{OH} } - C{H_2} - CHO\buildrel \Delta \over \longrightarrow $$
Which of these reaction(s) will not produce Saytzeff product ?
$${\left( {C{H_3}} \right)_3}CCH\left( {OH} \right)C{H_3}\buildrel {conc.\,\,\,{H_2}S{O_4}} \over \longrightarrow $$
$${\left( {C{H_3}} \right)_2}CHCH\left( {Br} \right)C{H_3}\buildrel {alc.\,\,KOH} \over \longrightarrow $$
$${\left( {C{H_3}} \right)_2}{\rm{ }}CHCH\left( {Br} \right)C{H_3}\buildrel {{{(C{H_3})}_3}{O^\Theta }{K^ \oplus }} \over \longrightarrow $$
$${\left( {C{H_3}} \right)_2}\mathop C\limits_{\mathop |\limits_{OH} } - C{H_2} - CHO\buildrel \Delta \over \longrightarrow $$
Which of these reaction(s) will not produce Saytzeff product ?
10
The dipole of CCl4, CHCl3 and CH4 are in the order :
11
Amongst the following statements, that which was not proposed by Dalton was :
12
Match the following :
(i) Riboflavin (a) Beriberi
(ii) Thiamine (b) Scurvy
(iii) Pyridoxine (c) Cheliosis
(iv) Ascorbic acid (d) Convulsions
(i) Riboflavin (a) Beriberi
(ii) Thiamine (b) Scurvy
(iii) Pyridoxine (c) Cheliosis
(iv) Ascorbic acid (d) Convulsions
13
The relative strength of interionic/intermolecular forces in decreasing order is :
14
The IUPAC name of complex [Pt(NH3)2Cl(NH2CH3)]Cl is:
15
The electron gain enthalpy (in KJ/mol) of fluorine, chlorine, bromine and iodine, respectively are:
16
1- methyl ethylene oxide when treated with an excess of HBr produces :
17
A solution of m-chloronailimne, m-chlorophenol and m-chlorobenzoic acid in ethyal acetate was extracted initially with a saturated solution of NaHCO3
to give fraction A. The left over organic phase
was extracted with dilute NaoH solution tio give fraction B. The final organiic layer was labelled as
fraction C. Fractions A,B and C, contain respectively :
18
At 35oC, the vapour pressure of CS2
is 512 mm. Hg and that of acetone is 344 mm Hg. A solution
of CS2
in acetone has a total vapour pressure of 600 mm Hg. The false statement amongst the
following is :
19
The increasing order to Pkb for the following compounds will be :


20
Oxidation number of potassium in K2O. K2O2 and KO2 respectively is :
21
The theory that can completely/properly explain the nature of bonding in [Ni(Co)4] is :
22
The atomic radius of Ag is closed to :
Mathematics
1
Total number of 6-digit numbers in which only and all the five digits 1, 3, 5, 7 and 9 appear, is :
2
Let $$\alpha $$ and $$\beta $$ be two real roots of the equation
(k + 1)tan2x - $$\sqrt 2 $$ . $$\lambda $$tanx = (1 - k), where k($$ \ne $$ - 1) and $$\lambda $$ are real numbers. if tan2 ($$\alpha $$ + $$\beta $$) = 50, then a value of $$\lambda $$ is:
(k + 1)tan2x - $$\sqrt 2 $$ . $$\lambda $$tanx = (1 - k), where k($$ \ne $$ - 1) and $$\lambda $$ are real numbers. if tan2 ($$\alpha $$ + $$\beta $$) = 50, then a value of $$\lambda $$ is:
3
If $${\mathop{\rm Re}\nolimits} \left( {{{z - 1} \over {2z + i}}} \right) = 1$$, where z = x + iy, then the point (x, y) lies on a :
4
A vector $$\overrightarrow a = \alpha \widehat i + 2\widehat j + \beta \widehat k\left( {\alpha ,\beta \in R} \right)$$ lies in the plane of the vectors, $$\overrightarrow b = \widehat i + \widehat j$$ and $$\overrightarrow c = \widehat i - \widehat j + 4\widehat k$$. If $$\overrightarrow a $$ bisects the angle between $$\overrightarrow b $$ and $$\overrightarrow c $$, then:
5
Let $$\alpha $$ be a root of the equation x2 + x + 1 = 0 and the
matrix A = $${1 \over {\sqrt 3 }}\left[ {\matrix{ 1 & 1 & 1 \cr 1 & \alpha & {{\alpha ^2}} \cr 1 & {{\alpha ^2}} & {{\alpha ^4}} \cr } } \right]$$
then the matrix A31 is equal to
matrix A = $${1 \over {\sqrt 3 }}\left[ {\matrix{ 1 & 1 & 1 \cr 1 & \alpha & {{\alpha ^2}} \cr 1 & {{\alpha ^2}} & {{\alpha ^4}} \cr } } \right]$$
then the matrix A31 is equal to
6
Let xk + yk = ak, (a, k > 0 ) and $${{dy} \over {dx}} + {\left( {{y \over x}} \right)^{{1 \over 3}}} = 0$$, then k is:
7
If the system of linear equations
2x + 2ay + az = 0
2x + 3by + bz = 0
2x + 4cy + cz = 0,
where a, b, c $$ \in $$ R are non-zero distinct; has a non-zero solution, then:
2x + 2ay + az = 0
2x + 3by + bz = 0
2x + 4cy + cz = 0,
where a, b, c $$ \in $$ R are non-zero distinct; has a non-zero solution, then:
8
$$\mathop {\lim }\limits_{x \to 2} {{{3^x} + {3^{3 - x}} - 12} \over {{3^{ - x/2}} - {3^{1 - x}}}}$$ is equal to_______.
9
If the variance of the first n natural numbers is 10 and the variance of the first m even natural
numbers is 16, then m + n is equal to_____.
10
Let A(1, 0), B(6, 2) and C $$\left( {{3 \over 2},6} \right)$$ be the vertices of a triangle ABC. If P is a Point inside the triangle ABC such that the triangles APC, APB and BPC have equal areas, then the length of the line segment PQ, where Q is the point $$\left( { - {7 \over 6}, - {1 \over 3}} \right)$$, is ________.
11
Let S be the set of points where the function, ƒ(x) = |2-|x-3||, x $$ \in $$ R is not differentiable. Then $$\sum\limits_{x \in S} {f(f(x))} $$ is equal to_____.
12
Five numbers are in A.P. whose sum is 25 and product is 2520. If one of these five numbers is -$${1 \over 2}$$ , then the greatest number amongst them is:
13
If y = y(x) is the solution of the differential equation, $${e^y}\left( {{{dy} \over {dx}} - 1} \right) = {e^x}$$ such that y(0) = 0, then
y(1) is equal to:
14
If $$y\left( \alpha \right) = \sqrt {2\left( {{{\tan \alpha + \cot \alpha } \over {1 + {{\tan }^2}\alpha }}} \right) + {1 \over {{{\sin }^2}\alpha }}} ,\alpha \in \left( {{{3\pi } \over 4},\pi } \right)$$
$${{dy} \over {d\alpha }}\,\,at\,\alpha = {{5\pi } \over 6}is$$ :
$${{dy} \over {d\alpha }}\,\,at\,\alpha = {{5\pi } \over 6}is$$ :
15
If g(x) = x2 + x - 1 and
(goƒ) (x) = 4x2 - 10x + 5, then ƒ$$\left( {{5 \over 4}} \right)$$ is equal to:
(goƒ) (x) = 4x2 - 10x + 5, then ƒ$$\left( {{5 \over 4}} \right)$$ is equal to:
16
If ƒ(a + b + 1 - x) = ƒ(x), for all x, where a and b are fixed positive real numbers, then
$${1 \over {a + b}}\int_a^b {x\left( {f(x) + f(x + 1)} \right)} dx$$ is equal to:
$${1 \over {a + b}}\int_a^b {x\left( {f(x) + f(x + 1)} \right)} dx$$ is equal to:
17
The greatest positive integer k, for which 49k + 1 is a factor of the sum
49125 + 49124 + ..... + 492 + 49 + 1, is:
49125 + 49124 + ..... + 492 + 49 + 1, is:
18
An unbiased coin is tossed 5 times. Suppose that a variable X is assigned the value of k when k
consecutive heads are obtained for k = 3, 4, 5, otherwise X takes the value -1. Then the expected
value of X, is :
19
If the distance between the foci of an ellipse is 6 and the distance between its directrices is 12,
then the length of its latus rectum is :
20
The area of the region, enclosed by the circle x2 + y2 = 2 which is not common to the region bounded by the parabola y2 = x and the straight line y = x, is:
Physics
1
The time period of revolution of electron in its ground state orbit in a hydrogen atom is 1.6 $$ \times $$ 10-16 s. The frequency of revolution of the electron in its first excited state (in s-1) is :
2
A beam of electromagnetic radiation of intensity 6.4 × 10–5 W/cm2 is comprised of wavelength,
$$\lambda $$ = 310 nm. It falls normally on a metal (work function $$\phi $$ = 2eV) of surface area of 1 cm2. If one
in 103 photons ejects an elctron, total number of electrons ejected in 1 s is 10x. (hc = 1240 eVnm,
1eV = 1.6 × 10–19 J), then x is _____.
3
A litre of dry air at STP expands adiabatically to a volume of 3 litres. If $$\gamma $$ = 1.40, the work done by air is : (31.4 = 4.6555) [Take air to be an ideal gas]
4
A particle (m = 1 kg) slides down a frictionless track (AOC) starting from rest at a point A (height 2 m). After reaching C, the particle continues to move freely in air as a projectile. When it reaching its highest point P (height 1 m), the kinetic energy of the particle (in )) is :
(Figure drawn is schematic and not to scale; take g = 10 ms-2)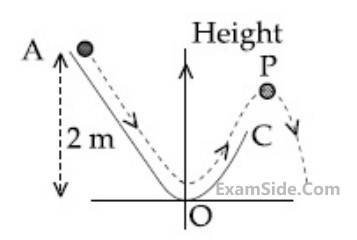
(Figure drawn is schematic and not to scale; take g = 10 ms-2)

5
 A parallel plate capacitor has plates of area A separated by distance 'd' between them. It is filled with a dielectric which has a dielectric constant that varies as k(x) = K(1 + $$\alpha $$x) where 'x' is the distance measured from one of the plates. If (ad) << 1, the total capacitance of the system is best given by the expression :
A parallel plate capacitor has plates of area A separated by distance 'd' between them. It is filled with a dielectric which has a dielectric constant that varies as k(x) = K(1 + $$\alpha $$x) where 'x' is the distance measured from one of the plates. If (ad) << 1, the total capacitance of the system is best given by the expression :6
If the magnetic field in a plane electromagnetic wave is given by
$$\overrightarrow B $$ = 3 $$ \times $$ 10-8 sin(1.6 $$ \times $$ 103x + 48 $$ \times $$ 1010t)$$\widehat j$$ T, then what will be expression for electric field ?
$$\overrightarrow B $$ = 3 $$ \times $$ 10-8 sin(1.6 $$ \times $$ 103x + 48 $$ \times $$ 1010t)$$\widehat j$$ T, then what will be expression for electric field ?
7
The current I1 (in A) flowing through 1 $$\Omega $$ resistor in the following circuit is :


8
Which of the following gives a reversible operation?
9
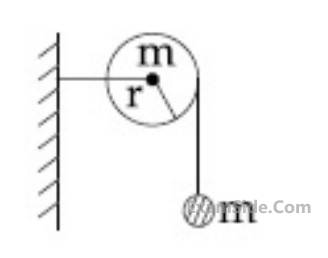 As shown in the figure, a bob of mass m is tied by a massless string whose other end portion is wound on a fly wheel (disc) of radius r and mass m. When released from rest the bob starts falling vertically. When it has covered a distance of h, the angular speed of the wheel will be:
As shown in the figure, a bob of mass m is tied by a massless string whose other end portion is wound on a fly wheel (disc) of radius r and mass m. When released from rest the bob starts falling vertically. When it has covered a distance of h, the angular speed of the wheel will be:10
If we need a magnification of 375 from a compound microscope of tube length 150 mm and an objective of focal length 5 mm, the focal length of the eye-piece, should be close to :
11
A LCR circuit behaves like a damped harmonic oscillator. Comparing it with a physical spring-mass damped oscillator having damping constant 'b', the correct equivalence would be:
12
A satellite of mass m is launched vertically upwards with an initial speed u from the surface of the earth. After it reaches height R (R = radius of the earth), it ejects a rocket
of mass $${m \over {10}}$$
so that subsequently the
satellite moves in a circular orbit. The kinetic energy of the rocket is (G is the gravitational constant; M is the mass of the earth) :
13
Speed of a transverse wave on a straight wire (mass 6.0 g, length 60 cm and area of cross-section 1.0 mm2) is 90 ms-1. If the Young's modulus of wire is 16 $$ \times $$ 1011 Nm-2, the extension of wire over its natural length is :
14
Two infinite planes each with uniform surface charge density to are kept in such a way that the angle between them is 30o. The electric field in the region shown between them is given by :


15
Two moles of an ideal gas with $${{{C_P}} \over {{C_V}}} = {5 \over 3}$$
are mixed with 3 moles of another ideal gas
with $${{{C_P}} \over {{C_V}}} = {4 \over 3}$$. The value of $${{{C_P}} \over {{C_V}}}$$ for the
mixture is :
16
A long solenoid of radius R carries a time (t) - dependent current
I(t)=I0t(1 - t). A ring of radius 2R is placed coaxially near its middle. During the time interval 0 $$ \le $$ t $$ \le $$ 1, the induced current (IR) and the induced EMF(VR) in the ring change as :
I(t)=I0t(1 - t). A ring of radius 2R is placed coaxially near its middle. During the time interval 0 $$ \le $$ t $$ \le $$ 1, the induced current (IR) and the induced EMF(VR) in the ring change as :
17
The radius of gyration of a uniform rod of length $$l$$, about an axis passing through a
point $${l \over 4}$$ away from the centre of the rod,
and perpendicular to it, is :
18
A polarizer - analyser set is adjusted such that the intensity of light coming out of the analyser is just 10% of the original intensity. Assuming that the polarizer - analyser set does not absorb any light, the angle by which the analyser need to be rotated further to reduce the output intensity to be
zero, is :
19
Consider a circular coil of wire carrying constant current I, forming a magnetic dipole. The magnetic flux through an infinite plane that contains the circular coil and excluding the circular coil area is given by $$\phi $$i. The magnetic flux through the area of the circular coil area is given by $$\phi $$0. Which of the following option is correct?
20
A 60 HP electric motor lifts an elevator having a maximum total load capacity of 2000 kg. If the frictional force on the elevator is 4000 N, the speed of the elevator at full load is close to :
(1 HP = 746 W, g = 10 ms-2)
(1 HP = 746 W, g = 10 ms-2)
21
A loop ABCDEFA of straight edges has six corner points A(0, 0, 0), B(5, 0, 0), C(5, 5, 0), D (0, 5,
0), E(0, 5, 5) and F(0, 0, 5). The magnetic field in this region is $$\overrightarrow B = \left( {3\widehat i + 4\widehat k} \right)T$$
. The quantity of
flux through the loop ABCDEFA (in Wb) is _______.
22
Visible light of wavelength 6000 $$ \times $$ 10-8 cm falls normally on a single slit and produces a diffraction pattern. It is found that the second diffraction minimum is at 60o from the central maximum. If the first minimum is produced at $$\theta $$1, then $$\theta $$1, is close to :
23
A non-isotropic solid metal cube has coefficients of linear expansion as :
5 $$ \times $$ 10-5/oC along the x-axis and 5 $$ \times $$ 10-6/oC along the y and the z-axis. If the coefficient of volume expansion of the solid is C $$ \times $$ 10-6/oC then the value of C is
5 $$ \times $$ 10-5/oC along the x-axis and 5 $$ \times $$ 10-6/oC along the y and the z-axis. If the coefficient of volume expansion of the solid is C $$ \times $$ 10-6/oC then the value of C is
24
Three point particles of masses 1.0 kg, 1.5 kg and 2.5 kg are placed at three corners of a right angle triangle of sides 4.0 cm, 3.0 cm and 5.0 cm as shown in the figure. The center of mass of the system is at a point:

