JEE Main 2021 (Online) 25th February Evening Shift
Paper was held on
Thu, Feb 25, 2021 9:30 AM
Chemistry
1
The correct sequence of reagents used in the preparation of 4-bromo-2-nitroethyl benzene from benzene is :
2
What is 'X' in the given reaction?


3
The solubility of Ca(OH)2 in water is :
[Given : The solubility product of Ca(OH)2 in water = 5.5 $$\times$$ 10$$-$$6]
[Given : The solubility product of Ca(OH)2 in water = 5.5 $$\times$$ 10$$-$$6]
4
Which among the following species has unequal bond lengths?
5
In which of the following order the given complex ions are arranged correctly with respect to their decreasing spin only magnetic moment?
(i) [FeF6]3$$-$$
(ii) [Co(NH3)6]3+
(iii) [NiCl4]2$$-$$
(iv) [Cu(NH3)4]2+
(i) [FeF6]3$$-$$
(ii) [Co(NH3)6]3+
(iii) [NiCl4]2$$-$$
(iv) [Cu(NH3)4]2+
6
The correct order of acid character of the following compounds is :


7
The major product of the following reaction is :


8

Correct statement about the given chemical reaction is :
9
Given below are two statements :
Statement I : The identification of Ni2+ is carried out by dimethyl glyoxime in the presence of NH4OH.
Statement II : The dimethyl glyoxime is a bidentate neutral ligand.
In the light of the above statements, choose the correct answer from the options given below :
Statement I : The identification of Ni2+ is carried out by dimethyl glyoxime in the presence of NH4OH.
Statement II : The dimethyl glyoxime is a bidentate neutral ligand.
In the light of the above statements, choose the correct answer from the options given below :
10
Carbylamine test is used to detect the presence of primary amino group in an organic compound. Which of the following compound is formed when this test is performed with aniline?
11
Which of the following is correct structure of $$\alpha$$-anomer of maltose?
12
Which of the following compound is added to the sodium extract before addition of silver nitrate for testing of halogens?
13
The major product of the following reaction is :


14
The correct order of bond dissociation enthalpy of halogens is :
15
Copper reduces NO$$_3^ - $$ into NO and NO2 depending upon the concentration of HNO3 in solution. (Assuming fixed [Cu2+] and PNO = PNO2), the HNO3 concentration at which the thermodynamic tendency for reduction of NO$$_3^ - $$ into NO and NO2 by copper is same is 10x M. The value of 2x is _______. (Rounded off to the nearest integer)
[Given, $$E_{C{u^{2 + }}/Cu}^o = 0.34$$ V, $$E_{NO_3^ - /NO}^o = 0.96$$ V, $$E_{NO_3^ - /N{O_2}}^o = 0.79$$ V and at 298 K, $${{RT} \over F}$$(2.303) = 0.059]
[Given, $$E_{C{u^{2 + }}/Cu}^o = 0.34$$ V, $$E_{NO_3^ - /NO}^o = 0.96$$ V, $$E_{NO_3^ - /N{O_2}}^o = 0.79$$ V and at 298 K, $${{RT} \over F}$$(2.303) = 0.059]
16
The spin only magnetic moment of a divalent ion in aqueous solution (atomic number 29) is _________ BM.
17
If a compound AB dissociates to the extent of 75% in an aqueous solution, the molality of the solution which shows a 2.5 K rise in the boiling point of the solution is ____________ molal. (Rounded off to the nearest integer)
[Kb = 0.52 K kg mol$$-$$1]
[Kb = 0.52 K kg mol$$-$$1]
18
Electromagnetic radiation of wavelength 663 nm is just sufficient to ionise the atom of metal A. The ionization enegy of metal A in kJ mol$$-$$1 is __________. (Rounded off to the nearest integer)
[h = 6.63 $$\times$$ 10$$-$$34 Js, c = 3.00 $$\times$$ 108 ms$$-$$1, NA = 6.02 $$\times$$ 1023 mol$$-$$1]
[h = 6.63 $$\times$$ 10$$-$$34 Js, c = 3.00 $$\times$$ 108 ms$$-$$1, NA = 6.02 $$\times$$ 1023 mol$$-$$1]
19
Consider titration of NaOH solution versus 1.25 M oxalic acid solution. At the end point following burette readings were obtained.
(i) 4.5 mL
(ii) 4.5 mL
(iii) 4.4 mL
(iv) 4.4 mL
(v) 4.4 mL
If the volume of oxalic acid taken was 10.0 mL then the molarity of the NaOH solution is ________ M. (Rounded off to the nearest integer)
(i) 4.5 mL
(ii) 4.5 mL
(iii) 4.4 mL
(iv) 4.4 mL
(v) 4.4 mL
If the volume of oxalic acid taken was 10.0 mL then the molarity of the NaOH solution is ________ M. (Rounded off to the nearest integer)
20
Five moles of an ideal gas at 293 K is expanded isothermally from an initial pressure of 2.1 MPa to 1.3 MPa against at constant external pressure 4.3 MPa. The heat transferred in this process is _________ kJ mol$$-$$1. (Rounded off to the nearest integer) [Use R = 8.314 J mol$$-$$1K$$-$$1]
21
The number of compound/s given below which contain/s -COOH group is __________. (Integer answer)
(A) Sulphanilic acid
(B) Picric acid
(C) Aspirin
(D) Ascorbic acid
(A) Sulphanilic acid
(B) Picric acid
(C) Aspirin
(D) Ascorbic acid
22
The rate constant of a reaction increases by five times on increase in temperature from 27$$^\circ$$C to 52$$^\circ$$C. The value of activation energy in kJ mol$$-$$1 is _________. (Rounded off to the nearest integer)
[R = 8.314 J K$$-$$1mol$$-$$1]
[R = 8.314 J K$$-$$1mol$$-$$1]
23
Among the following, number of metal/s which can be used as electrodes in the photoelectric cell is _________. (Integer answer)
(A) Li
(B) Na
(C) Rb
(D) Cs
(A) Li
(B) Na
(C) Rb
(D) Cs
Mathematics
1
The shortest distance between the line x $$-$$ y = 1 and the curve x2 = 2y is :
2
A function f(x) is given by $$f(x) = {{{5^x}} \over {{5^x} + 5}}$$, then the sum of the series $$f\left( {{1 \over {20}}} \right) + f\left( {{2 \over {20}}} \right) + f\left( {{3 \over {20}}} \right) + ....... + f\left( {{{39} \over {20}}} \right)$$ is equal to :
3
Let x denote the total number of one-one functions from a set A with 3 elements to a set B with 5 elements and y denote the total number of one-one functions form the set A to the set A $$\times$$ B. Then :
4
The integral $$\int {{{{e^{3{{\log }_e}2x}} + 5{e^{2{{\log }_e}2x}}} \over {{e^{4{{\log }_e}x}} + 5{e^{3{{\log }_e}x}} - 7{e^{2{{\log }_e}x}}}}} dx$$, x > 0, is equal to : (where c is a constant of integration)
5
Let A be a set of all 4-digit natural numbers whose exactly one digit is 7. Then the probability that a randomly chosen element of A leaves remainder 2 when divided by 5 is :
6
Let $$\alpha$$ and $$\beta$$ be the roots of x2 $$-$$ 6x $$-$$ 2 = 0. If an = $$\alpha$$n $$-$$ $$\beta$$n for n $$ \ge $$ 1, then the value of $${{{a_{10}} - 2{a_8}} \over {3{a_9}}}$$ is :
7
If 0 < x, y < $$\pi$$ and cosx + cosy $$-$$ cos(x + y) = $${3 \over 2}$$, then sinx + cosy is equal to :
8
cosec$$\left[ {2{{\cot }^{ - 1}}(5) + {{\cos }^{ - 1}}\left( {{4 \over 5}} \right)} \right]$$ is equal to :
9
Let A be a 3 $$\times$$ 3 matrix with det(A) = 4. Let Ri denote the ith row of A. If a matrix B is obtained by performing the operation R2 $$ \to $$ 2R2 + 5R3 on 2A, then det(B) is equal to :
10
If $$\alpha$$, $$\beta$$ $$\in$$ R are such that 1 $$-$$ 2i (here i2 = $$-$$1) is a root of z2 + $$\alpha$$z + $$\beta$$ = 0, then ($$\alpha$$ $$-$$ $$\beta$$) is equal to :
11
The minimum value of $$f(x) = {a^{{a^x}}} + {a^{1 - {a^x}}}$$, where a, $$x \in R$$ and a > 0, is equal to :
12
If $${I_n} = \int\limits_{{\pi \over 4}}^{{\pi \over 2}} {{{\cot }^n}x\,dx} $$, then :
13
In a group of 400 people, 160 are smokers and non-vegetarian; 100 are smokers and vegetarian and the remaining 140 are non-smokers and vegetarian. Their chances of getting a particular chest disorder are 35%, 20% and 10% respectively. A person is chosen from the group at random and is found to be suffering from the chest disorder. The probability that the selected person is a smoker and non-vegetarian is :
14
If for the matrix, $$A = \left[ {\matrix{
1 & { - \alpha } \cr
\alpha & \beta \cr
} } \right]$$, $$A{A^T} = {I_2}$$, then the value of $${\alpha ^4} + {\beta ^4}$$ is :
15
If the curve x2 + 2y2 = 2 intersects the line x + y = 1 at two points P and Q, then the angle subtended by the line segment PQ at the origin is :
16
The following system of linear equations
2x + 3y + 2z = 9
3x + 2y + 2z = 9
x $$-$$ y + 4z = 8
2x + 3y + 2z = 9
3x + 2y + 2z = 9
x $$-$$ y + 4z = 8
17
A hyperbola passes through the foci of the ellipse $${{{x^2}} \over {25}} + {{{y^2}} \over {16}} = 1$$ and its transverse and conjugate axes coincide with major and minor axes of the ellipse, respectively. If the product of their eccentricities is one, then the equation of the hyperbola is :
18
If the remainder when x is divided by 4 is 3, then the remainder when (2020 + x)2022 is divided by 8 is __________.
19
The total number of two digit numbers 'n', such that 3n + 7n is a multiple of 10, is __________.
20
The value of $$\int\limits_{ - 2}^2 {|3{x^2} - 3x - 6|dx} $$ is ___________.
21
A line 'l' passing through origin is perpendicular to the lines
$${l_1}:\overrightarrow r = (3 + t)\widehat i + ( - 1 + 2t)\widehat j + (4 + 2t)\widehat k$$
$${l_2}:\overrightarrow r = (3 + 2s)\widehat i + (3 + 2s)\widehat j + (2 + s)\widehat k$$
If the co-ordinates of the point in the first octant on 'l2‘ at a distance of $$\sqrt {17} $$ from the point of intersection of 'l' and 'l1' are (a, b, c) then 18(a + b + c) is equal to ___________.
$${l_1}:\overrightarrow r = (3 + t)\widehat i + ( - 1 + 2t)\widehat j + (4 + 2t)\widehat k$$
$${l_2}:\overrightarrow r = (3 + 2s)\widehat i + (3 + 2s)\widehat j + (2 + s)\widehat k$$
If the co-ordinates of the point in the first octant on 'l2‘ at a distance of $$\sqrt {17} $$ from the point of intersection of 'l' and 'l1' are (a, b, c) then 18(a + b + c) is equal to ___________.
22
A function f is defined on [$$-$$3, 3] as
$$f(x) = \left\{ {\matrix{ {\min \{ |x|,2 - {x^2}\} ,} & { - 2 \le x \le 2} \cr {[|x|],} & {2 < |x| \le 3} \cr } } \right.$$ where [x] denotes the greatest integer $$ \le $$ x. The number of points, where f is not differentiable in ($$-$$3, 3) is ___________.
$$f(x) = \left\{ {\matrix{ {\min \{ |x|,2 - {x^2}\} ,} & { - 2 \le x \le 2} \cr {[|x|],} & {2 < |x| \le 3} \cr } } \right.$$ where [x] denotes the greatest integer $$ \le $$ x. The number of points, where f is not differentiable in ($$-$$3, 3) is ___________.
23
Let $$\overrightarrow a = \widehat i + \alpha \widehat j + 3\widehat k$$ and $$\overrightarrow b = 3\widehat i - \alpha \widehat j + \widehat k$$. If the area of the parallelogram whose adjacent sides are represented by the vectors $$\overrightarrow a $$ and $$\overrightarrow b $$ is $$8\sqrt 3 $$ square units, then $$\overrightarrow a $$ . $$\overrightarrow b $$ is equal to __________.
24
If $$\mathop {\lim }\limits_{x \to 0} {{ax - ({e^{4x}} - 1)} \over {ax({e^{4x}} - 1)}}$$ exists and is equal to b, then the value of a $$-$$ 2b is __________.
25
If the curve, y = y(x) represented by the solution of the differential equation (2xy2 $$-$$ y)dx + xdy = 0, passes through the intersection of the lines, 2x $$-$$ 3y = 1 and 3x + 2y = 8, then |y(1)| is equal to _________.
Physics
1
An LCR circuit contains resistance of 110$$\Omega$$ and a supply of 220 V at 300 rad/s angular frequency. If only capacitance is removed from the circuit, current lags behind the voltage by 45$$^\circ$$. If on the other hand, only inductor is removed the current leads by 45$$^\circ$$ with the applied voltage. The rms current flowing in the circuit will be :
2
An electron with kinetic energy K1 enters between parallel plates of a capacitor at an angle '$$\alpha$$' with the plates. It leaves the plates at angle '$$\beta$$' with kinetic energy K2. Then the ratio of kinetic energies K1 : K2 will be :
3
A sphere of radius 'a' and mass 'm' rolls along a horizontal plane with constant speed v0. It encounters an inclined plane at angle $$\theta$$ and climbs upward. Assuming that it rolls without slipping, how far up the sphere will travel?
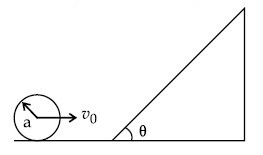

4
An electron of mass me and a proton of mass mp = 1836 me are moving with the same speed. The ratio of their de Broglie wavelength $${{{}^\lambda electron} \over {{}^\lambda proton}}$$ will be :
5
Consider the diffraction pattern obtained from the sunlight incident on a pinhole of diameter 0.1 $$\mu$$m. If the diameter of the pinhole is slightly increased, it will affect the diffraction pattern such that:
6
In a ferromagnetic material, below the curie temperature, a domain is defined as :
7
Given below are two statements :
Statement I : In a diatomic molecule, the rotational energy at a given temperature obeys Maxwell's distribution.
Statement II : In a diatomic molecule, the rotational energy at a given temperature equals the translational kinetic energy for each molecule.
In the light of the above statements, choose the correct answer from the options given below :
Statement I : In a diatomic molecule, the rotational energy at a given temperature obeys Maxwell's distribution.
Statement II : In a diatomic molecule, the rotational energy at a given temperature equals the translational kinetic energy for each molecule.
In the light of the above statements, choose the correct answer from the options given below :
8
Match List I with List II.
Choose the correct answer from the options given below :
| List I | List II | ||
|---|---|---|---|
| (a) | Rectifier | (i) | Used either for stepping up or stepping down the a.c. voltage |
| (b) | Stabilizer | (ii) | Used to convert a.c. voltage into d.c. voltage |
| (c) | Transformer | (iii) | Used to remove any ripple in the rectified output voltage |
| (d) | Filter | (iv) | Used for constant output voltage even when the input voltage or load current change |
Choose the correct answer from the options given below :
9
A charge 'q' is placed at one corner of a cube as shown in figure. The flux of electrostatic field $$\overrightarrow E $$ through the shaded area is :


10
Thermodynamic process is shown below on a P-V diagram for one mole of an ideal gas. If V2 = 2V1 then the ratio of temperature T2/T1 is :
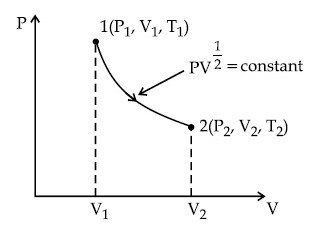

11
The wavelength of the photon emitted by a hydrogen atom when an electron makes a transition from n = 2 to n = 1 state is :
12
Y = A sin($$\omega$$t + $$\phi$$0) is the time-displacement equation of a SHM. At t = 0 the displacement of the particle is $$Y = {A \over 2}$$ and it is moving along negative x-direction. Then the initial phase angle $$\phi$$0 will be:
13
Two identical springs of spring constant '2k' are attached to a block of mass m and to fixed support (see figure). When the mass is displaced from equilibrium position on either side, it executes simple harmonic motion. The time period of oscillations of this system is :
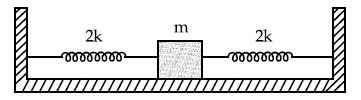

14
The point A moves with a uniform speed along the circumference of a circle of radius 0.36 m and covers 30$$^\circ$$ in 0.1 s. The perpendicular projection 'P' from 'A' on the diameter MN represents the simple harmonic motion of 'P'. The restoration force per unit mass when P touches M will be :
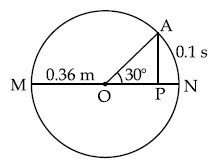

15
If e is the electronic charge, c is the speed of light in free space and h is Planck's constant, the quantity $${1 \over {4\pi {\varepsilon _0}}}{{|e{|^2}} \over {hc}}$$ has dimensions of :
16
For extrinsic semiconductors; when doping level is increased;
17
The truth table for the following logic circuit is :


18
A stone is dropped from the top of a building. When it crosses a point 5 m below the top, another stone starts to fall from a point 25 m below the top. Both stones reach the bottom of building simultaneously. The height of the building is :
19
The stopping potential for electrons emitted from a photosensitive surface illuminated by light of wavelength 491 nm is 0.710 V. When the incident wavelength is changed to a new value, the stopping potential is 1.43 V. The new wavelength is :
20
If $$\overrightarrow P \times \overrightarrow Q = \overrightarrow Q \times \overrightarrow P $$, the angle between $$\overrightarrow P $$ and $$\overrightarrow Q $$ is $$\theta$$(0$$^\circ$$ < $$\theta$$ < 360$$^\circ$$). The value of '$$\theta$$' will be ___________$$^\circ$$.
21
The percentage increase in the speed of transverse waves produced in a stretched string if the tension is increased by 4%, will be __________%.
22
The initial velocity vi required to project a body vertically upward from the surface of the earth to reach a height of 10R, where R is the radius of the earth, may be described in terms of escape velocity ve such that $${v_i} = \sqrt {{x \over y}} \times {v_e}$$. The value of x will be ____________.
23
The peak electric field produced by the radiation coming from the 8W bulb at a distance of 10 m is $${x \over {10}}\sqrt {{{{\mu _0}c} \over \pi }} {V \over m}$$. The efficiency of the bulb is 10% and it is a point source. The value of x is ___________.
24
Two identical conducting spheres with negligible volume have 2.1 nC and $$-$$0.1 nC charges, respectively. They are brought into contact and then separated by a distance of 0.5 m. The electrostatic force acting between the spheres is __________ $$\times$$ 10$$-$$9 N.
[Given : $$4\pi {\varepsilon _0} = {1 \over {9 \times {{10}^9}}}$$ SI unit]
[Given : $$4\pi {\varepsilon _0} = {1 \over {9 \times {{10}^9}}}$$ SI unit]
25
The wavelength of an X-ray beam is 10$$\mathop A\limits^o $$. The mass of a fictitious particle having the same energy as that of the X-ray photons is $${x \over 3}h$$ kg. The value of x is __________. (h = Planck's constant)
26
Two small spheres each of mass 10 mg are suspended from a point by threads 0.5 m long. They are equally charged and repel each other to a distance of 0.20 m. The charge on each of the sphere is $${a \over {21}} \times {10^{ - 8}}$$C. The value of 'a' will be ___________. [Given g = 10 ms$$-$$2]
27
Two particles having masses 4 g and 16 g respectively are moving with equal kinetic energies. The ratio of the magnitudes of their linear momentum is n : 2. The value of n will be ___________.
28
A current of 6A enters one corner P of an equilateral triangle PQR having 3 wires of resistance 2$$\Omega$$ each and leaves by the corner R. The currents i1 in ampere is _________.

