JEE Main 2024 (Online) 1st February Evening Shift
Paper was held on
Thu, Feb 1, 2024 9:30 AM
Chemistry
1
In the given reactions identify $A$ and $B$


2
Solubility of calcium phosphate (molecular mass, M) in water is $\mathrm{W_{g}}$ per $100 \mathrm{~mL}$ at $25^{\circ} \mathrm{C}$. Its solubility product at $25^{\circ} \mathrm{C}$ will be approximately.
3
Given below are two statements :
Statement (I) : $\mathrm{SiO}_2$ and $\mathrm{GeO}_2$ are acidic while $\mathrm{SnO}$ and $\mathrm{PbO}$ are amphoteric in nature.
Statement (II) : Allotropic forms of carbon are due to property of catenation and $\mathrm{p} \pi-\mathrm{d} \pi$ bond formation.
In the light of the above statements, choose the most appropriate answer from the options given below :
Statement (I) : $\mathrm{SiO}_2$ and $\mathrm{GeO}_2$ are acidic while $\mathrm{SnO}$ and $\mathrm{PbO}$ are amphoteric in nature.
Statement (II) : Allotropic forms of carbon are due to property of catenation and $\mathrm{p} \pi-\mathrm{d} \pi$ bond formation.
In the light of the above statements, choose the most appropriate answer from the options given below :
4
The set of meta directing functional groups from the following sets is :
5
$\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{3+}$ and $\left[\mathrm{CoF}_6\right]^{3-}$ are respectively known as :
6
The transition metal having highest $3^{\text {rd }}$ ionisation enthalpy is :
7
Match List - I with List - II.
Choose the correct answer from the options given below :
| List I (Compound) | List II (Use) |
|---|---|
| (A) Carbon tetrachloride | (I) Paint remover |
| (B) Methylene chloride | (II) Refrigerators and air conditioners |
| (C) DDT | (III) Fire extinguisher |
| (D) Freons | (IV) Non Biodegradable insecticide |
Choose the correct answer from the options given below :
8
Match List - I with List - II.
Choose the correct answer from the options given below :
| List I (Reactants) | List II (Product) |
|---|---|
| (A) Phenol, Zn/Δ | (I) Salicylaldehyde |
| (B) Phenol, CHCl3, NaOH, HCl | (II) Salicylic acid |
| (C) Phenol, CO2, NaOH, HCl | (III) Benzene |
| (D) Phenol, Conc. HNO3 | (IV) Picric acid |
Choose the correct answer from the options given below :
9
Given below are two statements :
Statement (I) : Dimethyl glyoxime forms a six-membered covalent chelate when treated with $\mathrm{NiCl}_2$ solution in presence of $\mathrm{NH}_4 \mathrm{OH}$.
Statement (II) : Prussian blue precipitate contains iron both in $(+2)$ and $(+3)$ oxidation states.
In the light of the above statements, choose the most appropriate answer from the options given below :
Statement (I) : Dimethyl glyoxime forms a six-membered covalent chelate when treated with $\mathrm{NiCl}_2$ solution in presence of $\mathrm{NH}_4 \mathrm{OH}$.
Statement (II) : Prussian blue precipitate contains iron both in $(+2)$ and $(+3)$ oxidation states.
In the light of the above statements, choose the most appropriate answer from the options given below :
10

Acid D formed in above reaction is :
11
Lassaigne's test is used for detection of :
12
The strongest reducing agent among the following is :
13
Given below are two statements : one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A) : In aqueous solutions $\mathrm{Cr}^{2+}$ is reducing while $\mathrm{Mn}^{3+}$ is oxidising in nature.
Reason (R) : Extra stability to half filled electronic configuration is observed than incompletely filled electronic configuration.
In the light of the above statements, choose the most appropriate answer from the options given below :
Assertion (A) : In aqueous solutions $\mathrm{Cr}^{2+}$ is reducing while $\mathrm{Mn}^{3+}$ is oxidising in nature.
Reason (R) : Extra stability to half filled electronic configuration is observed than incompletely filled electronic configuration.
In the light of the above statements, choose the most appropriate answer from the options given below :
14
The functional group that shows negative resonance effect is :
15
The number of radial node/s for $3 p$ orbital is :
16
Which among the followng has highest boiling point?
17
Given below are two statements :
Statement (I) : A $\pi$ bonding MO has lower electron density above and below the inter-nuclear axis.
Statement (II) : The $\pi^*$ antibonding MO has a node between the nuclei.
In the light of the above statements, choose the most appropriate answer from the options given below :
Statement (I) : A $\pi$ bonding MO has lower electron density above and below the inter-nuclear axis.
Statement (II) : The $\pi^*$ antibonding MO has a node between the nuclei.
In the light of the above statements, choose the most appropriate answer from the options given below :
18
Given below are two statements :
Statement (I) : Both metals and non-metals exist in p and d-block elements.
Statement (II) : Non-metals have higher ionisation enthalpy and higher electronegativity than the metals.
In the light of the above statements, choose the most appropriate answer from the options given below :
Statement (I) : Both metals and non-metals exist in p and d-block elements.
Statement (II) : Non-metals have higher ionisation enthalpy and higher electronegativity than the metals.
In the light of the above statements, choose the most appropriate answer from the options given below :
19
Which of the following compounds show colour due to d-d transition?
20
Select the compound from the following that will show intramolecular hydrogen bonding.
21
The number of tripeptides formed by three different amino acids using each amino acid once is ______.
22
Mass of ethylene glycol (antifreeze) to be added to $18.6 \mathrm{~kg}$ of water to protect the freezing point at $-24^{\circ} \mathrm{C}$ is ________ $\mathrm{kg}$ (Molar mass in $\mathrm{g} ~\mathrm{mol}^{-1}$ for ethylene glycol $62, \mathrm{~K}_f$ of water $=1.86 \mathrm{~K}$ $\mathrm{kg} ~\mathrm{mol}^{-1}$ )
23
Total number of isomeric compounds (including stereoisomers) formed by monochlorination of 2-methylbutane is _______ .
24
The following data were obtained during the first order thermal decomposition of a gas A at constant volume :
$\mathrm{A}(\mathrm{g}) \rightarrow 2 \mathrm{~B}(\mathrm{~g})+\mathrm{C}(\mathrm{g})$
The rate constant of the reaction is ________ $\times 10^{-2} \mathrm{~s}^{-1}$ (nearest integer)
$\mathrm{A}(\mathrm{g}) \rightarrow 2 \mathrm{~B}(\mathrm{~g})+\mathrm{C}(\mathrm{g})$
| S.No. | Time /s | Total pressure /(atm) |
|---|---|---|
| 1. | 0 | 0.1 |
| 2. | 115 | 0.28 |
The rate constant of the reaction is ________ $\times 10^{-2} \mathrm{~s}^{-1}$ (nearest integer)
25
For a certain reaction at $300 \mathrm{~K}, \mathrm{~K}=10$, then $\Delta \mathrm{G}^{\circ}$ for the same reaction is - ____________ $\times 10^{-1} \mathrm{~kJ} \mathrm{~mol}^{-1}$.
(Given $\mathrm{R}=8.314 \mathrm{JK}^{-1} \mathrm{~mol}^{-1}$ )
(Given $\mathrm{R}=8.314 \mathrm{JK}^{-1} \mathrm{~mol}^{-1}$ )
26
The amount of electricity in Coulomb required for the oxidation of $1 \mathrm{~mol}$ of $\mathrm{H}_2 \mathrm{O}$ to $\mathrm{O}_2$ is __________ $\times 10^5 \mathrm{C}$.
27
Following Kjeldahl's method, $1 \mathrm{~g}$ of organic compound released ammonia, that neutralised $10 \mathrm{~mL}$ of $2 \mathrm{M} ~\mathrm{H}_2 \mathrm{SO}_4$. The percentage of nitrogen in the compound is ___________ $\%$.
28
Consider the following redox reaction :
$$ \mathrm{MnO}_4^{-}+\mathrm{H}^{+}+\mathrm{H}_2 \mathrm{C}_2 \mathrm{O}_4 \rightleftharpoons \mathrm{Mn}^{2+}+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2 $$
The standard reduction potentials are given as below $\left(\mathrm{E}_{\text {red }}^0\right)$ :
$$ \begin{aligned} & \mathrm{E}_{\mathrm{MnO}_4^{-} / \mathrm{Mn}^{2+}}^{\circ}=+1.51 \mathrm{~V} \\\\ & \mathrm{E}_{\mathrm{CO}_2 / \mathrm{H}_2 \mathrm{C}_2 \mathrm{O}_4}^{\circ}=-0.49 \mathrm{~V} \end{aligned} $$
If the equilibrium constant of the above reaction is given as $\mathrm{K}_{\mathrm{eq}}=10^x$, then the value of $x=$ __________ (nearest integer)
$$ \mathrm{MnO}_4^{-}+\mathrm{H}^{+}+\mathrm{H}_2 \mathrm{C}_2 \mathrm{O}_4 \rightleftharpoons \mathrm{Mn}^{2+}+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2 $$
The standard reduction potentials are given as below $\left(\mathrm{E}_{\text {red }}^0\right)$ :
$$ \begin{aligned} & \mathrm{E}_{\mathrm{MnO}_4^{-} / \mathrm{Mn}^{2+}}^{\circ}=+1.51 \mathrm{~V} \\\\ & \mathrm{E}_{\mathrm{CO}_2 / \mathrm{H}_2 \mathrm{C}_2 \mathrm{O}_4}^{\circ}=-0.49 \mathrm{~V} \end{aligned} $$
If the equilibrium constant of the above reaction is given as $\mathrm{K}_{\mathrm{eq}}=10^x$, then the value of $x=$ __________ (nearest integer)
29
Number of compounds which give reaction with Hinsberg's reagent is _________.


30
$10 \mathrm{~mL}$ of gaseous hydrocarbon on combustion gives $40 \mathrm{~mL}$ of $\mathrm{CO}_2(\mathrm{~g})$ and $50 \mathrm{~mL}$ of water vapour. Total number of carbon and hydrogen atoms in the hydrocarbon is _________ .
Mathematics
1
If the domain of the function
$f(x)=\frac{\sqrt{x^2-25}}{\left(4-x^2\right)}+\log _{10}\left(x^2+2 x-15\right)$ is $(-\infty, \alpha) \cup[\beta, \infty)$, then $\alpha^2+\beta^3$ is equal to :
$f(x)=\frac{\sqrt{x^2-25}}{\left(4-x^2\right)}+\log _{10}\left(x^2+2 x-15\right)$ is $(-\infty, \alpha) \cup[\beta, \infty)$, then $\alpha^2+\beta^3$ is equal to :
2
If $z$ is a complex number such that $|z| \leqslant 1$, then the minimum value of $\left|z+\frac{1}{2}(3+4 i)\right|$ is :
3
Consider a $\triangle A B C$ where $A(1,3,2), B(-2,8,0)$ and $C(3,6,7)$. If the angle bisector of $\angle B A C$ meets
the line $B C$ at $D$, then the length of the projection of the vector $\overrightarrow{A D}$ on the vector $\overrightarrow{A C}$ is :
4
Consider the relations $R_1$ and $R_2$ defined as $a R_1 b \Leftrightarrow a^2+b^2=1$ for all $a, b \in \mathbf{R}$ and $(a, b) R_2(c, d) \Leftrightarrow$ $a+d=b+c$ for all $(a, b),(c, d) \in \mathbf{N} \times \mathbf{N}$. Then :
5
Let the system of equations $x+2 y+3 z=5,2 x+3 y+z=9,4 x+3 y+\lambda z=\mu$ have infinite number of solutions. Then $\lambda+2 \mu$ is equal to :
6
If $\int\limits_0^{\frac{\pi}{3}} \cos ^4 x \mathrm{~d} x=\mathrm{a} \pi+\mathrm{b} \sqrt{3}$, where $\mathrm{a}$ and $\mathrm{b}$ are rational numbers, then $9 \mathrm{a}+8 \mathrm{b}$ is equal to :
7
Let $\alpha$ and $\beta$ be the roots of the equation $p x^2+q x-r=0$, where $p \neq 0$. If $p, q$ and $r$ be the consecutive terms of a non constant G.P. and $\frac{1}{\alpha}+\frac{1}{\beta}=\frac{3}{4}$, then the value of $(\alpha-\beta)^2$ is :
8
Let Ajay will not appear in JEE exam with probability $\mathrm{p}=\frac{2}{7}$, while both Ajay and Vijay will appear in the exam with probability $\mathrm{q}=\frac{1}{5}$. Then the probability, that Ajay will appear in the exam and Vijay will not appear is :
9
Let $\mathrm{P}$ be a point on the ellipse $\frac{x^2}{9}+\frac{y^2}{4}=1$. Let the line passing through $\mathrm{P}$ and parallel to $y$-axis meet the circle $x^2+y^2=9$ at point $\mathrm{Q}$ such that $\mathrm{P}$ and $\mathrm{Q}$ are on the same side of the $x$-axis. Then, the eccentricity of the locus of the point $R$ on $P Q$ such that $P R: R Q=4: 3$ as $P$ moves on the ellipse, is :
10
Consider 10 observations $x_1, x_2, \ldots, x_{10}$ such that $\sum\limits_{i=1}^{10}\left(x_i-\alpha\right)=2$ and $\sum\limits_{i=1}^{10}\left(x_i-\beta\right)^2=40$, where $\alpha, \beta$ are positive integers. Let the mean and the variance of the observations be $\frac{6}{5}$ and $\frac{84}{25}$ respectively. Then $\frac{\beta}{\alpha}$ is equal to :
11
Let $f(x)=\left|2 x^2+5\right| x|-3|, x \in \mathbf{R}$. If $\mathrm{m}$ and $\mathrm{n}$ denote the number of points where $f$ is not continuous and not differentiable respectively, then $\mathrm{m}+\mathrm{n}$ is equal to :
12
The number of solutions of the equation $4 \sin ^2 x-4 \cos ^3 x+9-4 \cos x=0 ; x \in[-2 \pi, 2 \pi]$ is :
13
Let the locus of the midpoints of the chords of the circle $x^2+(y-1)^2=1$ drawn from the origin intersect the line $x+y=1$ at $\mathrm{P}$ and $\mathrm{Q}$. Then, the length of $\mathrm{PQ}$ is :
14
Let $\alpha$ be a non-zero real number. Suppose $f: \mathbf{R} \rightarrow \mathbf{R}$ is a differentiable function such that $f(0)=2$ and $\lim\limits_{x \rightarrow-\infty} f(x)=1$. If $f^{\prime}(x)=\alpha f(x)+3$, for all $x \in \mathbf{R}$, then $f\left(-\log _{\mathrm{e}} 2\right)$ is equal to :
15
Let $\mathrm{P}$ and $\mathrm{Q}$ be the points on the line $\frac{x+3}{8}=\frac{y-4}{2}=\frac{z+1}{2}$ which are at a distance of 6 units from the point $\mathrm{R}(1,2,3)$. If the centroid of the triangle PQR is $(\alpha, \beta, \gamma)$, then $\alpha^2+\beta^2+\gamma^2$ is :
16
The value of $\int\limits_0^1\left(2 x^3-3 x^2-x+1\right)^{\frac{1}{3}} \mathrm{~d} x$ is equal to :
17
If the mirror image of the point $P(3,4,9)$ in the line
$\frac{x-1}{3}=\frac{y+1}{2}=\frac{z-2}{1}$ is $(\alpha, \beta, \gamma)$, then 14 $(\alpha+\beta+\gamma)$ is :
$\frac{x-1}{3}=\frac{y+1}{2}=\frac{z-2}{1}$ is $(\alpha, \beta, \gamma)$, then 14 $(\alpha+\beta+\gamma)$ is :
18
Let $S_n$ denote the sum of the first $n$ terms of an arithmetic progression. If $S_{10}=390$ and the ratio of the tenth and the fifth terms is $15: 7$, then $\mathrm{S}_{15}-\mathrm{S}_5$ is equal to :
19
Let $m$ and $n$ be the coefficients of seventh and thirteenth terms respectively
in the expansion of $\left(\frac{1}{3} x^{\frac{1}{3}}+\frac{1}{2 x^{\frac{2}{3}}}\right)^{18}$. Then $\left(\frac{\mathrm{n}}{\mathrm{m}}\right)^{\frac{1}{3}}$ is :
in the expansion of $\left(\frac{1}{3} x^{\frac{1}{3}}+\frac{1}{2 x^{\frac{2}{3}}}\right)^{18}$. Then $\left(\frac{\mathrm{n}}{\mathrm{m}}\right)^{\frac{1}{3}}$ is :
20
Let $f(x)=\left\{\begin{array}{l}x-1, x \text { is even, } \\ 2 x, \quad x \text { is odd, }\end{array} x \in \mathbf{N}\right.$.
If for some $\mathrm{a} \in \mathbf{N}, f(f(f(\mathrm{a})))=21$, then $\lim\limits_{x \rightarrow \mathrm{a}^{-}}\left\{\frac{|x|^3}{\mathrm{a}}-\left[\frac{x}{\mathrm{a}}\right]\right\}$, where $[t]$ denotes the greatest integer less than or equal to $t$, is equal to :
If for some $\mathrm{a} \in \mathbf{N}, f(f(f(\mathrm{a})))=21$, then $\lim\limits_{x \rightarrow \mathrm{a}^{-}}\left\{\frac{|x|^3}{\mathrm{a}}-\left[\frac{x}{\mathrm{a}}\right]\right\}$, where $[t]$ denotes the greatest integer less than or equal to $t$, is equal to :
21
Three points $\mathrm{O}(0,0), \mathrm{P}\left(\mathrm{a}, \mathrm{a}^2\right), \mathrm{Q}\left(-\mathrm{b}, \mathrm{b}^2\right), \mathrm{a}>0, \mathrm{~b}>0$, are on the parabola $y=x^2$. Let $\mathrm{S}_1$ be the area of the region bounded by the line $\mathrm{PQ}$ and the parabola, and $\mathrm{S}_2$ be the area of the triangle $\mathrm{OPQ}$. If the minimum value of $\frac{\mathrm{S}_1}{\mathrm{~S}_2}$ is $\frac{\mathrm{m}}{\mathrm{n}}, \operatorname{gcd}(\mathrm{m}, \mathrm{n})=1$, then $\mathrm{m}+\mathrm{n}$ is equal to __________.
22
The sum of squares of all possible values of $k$, for which area of the region bounded by the parabolas $2 y^2=\mathrm{k} x$ and $\mathrm{ky}^2=2(y-x)$ is maximum, is equal to :
23
If $y=\frac{(\sqrt{x}+1)\left(x^2-\sqrt{x}\right)}{x \sqrt{x}+x+\sqrt{x}}+\frac{1}{15}\left(3 \cos ^2 x-5\right) \cos ^3 x$, then $96 y^{\prime}\left(\frac{\pi}{6}\right)$ is equal to :
24
If $\frac{\mathrm{d} x}{\mathrm{~d} y}=\frac{1+x-y^2}{y}, x(1)=1$, then $5 x(2)$ is equal to __________.
25
Let $f:(0, \infty) \rightarrow \mathbf{R}$ and $\mathrm{F}(x)=\int\limits_0^x \mathrm{t} f(\mathrm{t}) \mathrm{dt}$. If $\mathrm{F}\left(x^2\right)=x^4+x^5$, then $\sum\limits_{\mathrm{r}=1}^{12} f\left(\mathrm{r}^2\right)$ is equal to ____________.
26
Let $A B C$ be an isosceles triangle in which $A$ is at $(-1,0), \angle A=\frac{2 \pi}{3}, A B=A C$ and $B$ is on the positve $x$-axis. If $\mathrm{BC}=4 \sqrt{3}$ and the line $\mathrm{BC}$ intersects the line $y=x+3$ at $(\alpha, \beta)$, then $\frac{\beta^4}{\alpha^2}$ is __________.
27
Let $A=I_2-2 M M^T$, where $M$ is a real matrix of order $2 \times 1$ such that the relation $M^T M=I_1$ holds. If $\lambda$ is a real number such that the relation $A X=\lambda X$ holds for some non-zero real matrix $X$ of order $2 \times 1$, then the sum of squares of all possible values of $\lambda$ is equal to __________.
28
Let $\overrightarrow{\mathrm{a}}=\hat{i}+\hat{j}+\hat{k}, \overrightarrow{\mathrm{b}}=-\hat{i}-8 \hat{j}+2 \hat{k}$ and $\overrightarrow{\mathrm{c}}=4 \hat{i}+\mathrm{c}_2 \hat{j}+\mathrm{c}_3 \hat{k}$ be three vectors such that $\overrightarrow{\mathrm{b}} \times \overrightarrow{\mathrm{a}}=\overrightarrow{\mathrm{c}} \times \overrightarrow{\mathrm{a}}$. If the angle between the vector $\overrightarrow{\mathrm{c}}$ and the vector $3 \hat{i}+4 \hat{j}+\hat{k}$ is $\theta$, then the greatest integer less than or equal to $\tan ^2 \theta$ is _______________.
29
If three successive terms of a G.P. with common ratio $\mathrm{r}(\mathrm{r}>1)$ are the lengths of the sides of a triangle and $[r]$ denotes the greatest integer less than or equal to $r$, then $3[r]+[-r]$ is equal to _____________.
30
The lines $\mathrm{L}_1, \mathrm{~L}_2, \ldots, \mathrm{L}_{20}$ are distinct. For $\mathrm{n}=1,2,3, \ldots, 10$ all the lines $\mathrm{L}_{2 \mathrm{n}-1}$ are parallel to each other and all the lines $L_{2 n}$ pass through a given point $P$. The maximum number of points of intersection of pairs of lines from the set $\left\{\mathrm{L}_1, \mathrm{~L}_2, \ldots, \mathrm{L}_{20}\right\}$ is equal to ___________.
Physics
1
From the statements given below :
(A) The angular momentum of an electron in $n^{\text {th }}$ orbit is an integral multiple of $\hbar$.
(B) Nuclear forces do not obey inverse square law.
(C) Nuclear forces are spin dependent.
(D) Nuclear forces are central and charge independent.
(E) Stability of nucleus is inversely proportional to the value of packing fraction.
Choose the correct answer from the options given below :
(A) The angular momentum of an electron in $n^{\text {th }}$ orbit is an integral multiple of $\hbar$.
(B) Nuclear forces do not obey inverse square law.
(C) Nuclear forces are spin dependent.
(D) Nuclear forces are central and charge independent.
(E) Stability of nucleus is inversely proportional to the value of packing fraction.
Choose the correct answer from the options given below :
2
A body of mass $4 \mathrm{~kg}$ experiences two forces $\vec{F}_1=5 \hat{i}+8 \hat{j}+7 \hat{k}$ and $\overrightarrow{\mathrm{F}}_2=3 \hat{i}-4 \hat{j}-3 \hat{k}$. The acceleration acting on the body is :
3
Monochromatic light of frequency $6 \times 10^{14} \mathrm{~Hz}$ is produced by a laser. The power emitted is $2 \times 10^{-3} \mathrm{~W}$.
How many photons per second on an average, are emitted by the source ?
(Given $\mathrm{h}=6.63 \times 10^{-34} \mathrm{Js}$ )
How many photons per second on an average, are emitted by the source ?
(Given $\mathrm{h}=6.63 \times 10^{-34} \mathrm{Js}$ )
4
$C_1$ and $C_2$ are two hollow concentric cubes enclosing charges $2 Q$ and $3 Q$ respectively as shown in figure. The ratio of electric flux passing through $C_1$ and $C_2$ is :


5
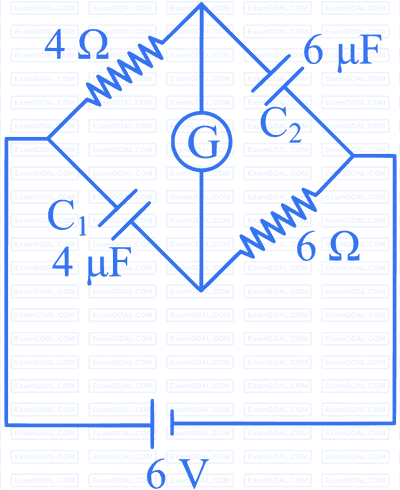
A galvanometer $(G)$ of $2 \Omega$ resistance is connected in the given circuit. The ratio of charge stored in $C_1$ and $C_2$ is :

6
Match List - I with List - II.
Choose the correct answer from the options given below :
| List I (Number) | List II (Significant figure) |
|---|---|
| (A) 1001 | (I) 3 |
| (B) 010.1 | (II) 4 |
| (C) 100.100 | (III) 5 |
| (D) 0.0010010 | (IV) 6 |
Choose the correct answer from the options given below :
7
A big drop is formed by coalescing 1000 small droplets of water. The surface energy will become :
8
A cricket player catches a ball of mass $120 \mathrm{~g}$ moving with $25 \mathrm{~m} / \mathrm{s}$ speed. If the catching process is completed in $0.1 \mathrm{~s}$ then the magnitude of force exerted by the ball on the hand of player will be (in SI unit) :
9
In a metre-bridge when a resistance in the left gap is $2 \Omega$ and unknown resistance in the right gap, the balance length is found to be $40 \mathrm{~cm}$. On shunting the unknown resistance with $2 \Omega$, the balance length changes by :
10
A diatomic gas $(\gamma=1.4)$ does $200 \mathrm{~J}$ of work when it is expanded isobarically. The heat given to the gas in the process is :
11
Train A is moving along two parallel rail tracks towards north with speed $$72 \mathrm{~km} / \mathrm{h}$$ and train B is moving towards south with speed $$108 \mathrm{~km} / \mathrm{h}$$. Velocity of train B with respect to A and velocity of ground with respect to B are (in $$\mathrm{ms}^{-1}$$):
12
A light planet is revolving around a massive star in a circular orbit of radius $\mathrm{R}$ with a period of revolution T. If the force of attraction between planet and star is proportional to $\mathrm{R}^{-3 / 2}$ then choose the correct option :
13
A microwave of wavelength $2.0 \mathrm{~cm}$ falls normally on a slit of width $4.0 \mathrm{~cm}$. The angular spread of the central maxima of the diffraction pattern obtained on a screen $1.5 \mathrm{~m}$ away from the slit, will be :
14
If the root mean square velocity of hydrogen molecule at a given temperature and pressure is $2 \mathrm{~km} / \mathrm{s}$, the root mean square velocity of oxygen at the same condition in $\mathrm{km} / \mathrm{s}$ is :
15
If frequency of electromagnetic wave is $60 \mathrm{~MHz}$ and it travels in air along $z$ direction then the corresponding electric and magnetic field vectors will be mutually perpendicular to each other and the wavelength of the wave (in $\mathrm{m}$ ) is :
16
To measure the temperature coefficient of resistivity $\alpha$ of a semiconductor, an electrical arrangement shown in the figure is prepared. The arm BC is made up of the semiconductor. The experiment is being conducted at $25^{\circ} \mathrm{C}$ and resistance of the semiconductor arm is $3 \mathrm{~m} \Omega$. Arm $\mathrm{BC}$ is cooled at a constant rate of $2^{\circ} \mathrm{C} / \mathrm{s}$. If the galvanometer $\mathrm{G}$ shows no deflection after $10 \mathrm{~s}$, then $\alpha$ is :


17
Conductivity of a photodiode starts changing only if the wavelength of incident light is less than $660 \mathrm{~nm}$. The band gap of photodiode is found to be $\left(\frac{\mathrm{X}}{8}\right) \mathrm{eV}$. The value of $\mathrm{X}$ is :
(Given, $\mathrm{h}=6.6 \times 10^{-34} \mathrm{Js}, \mathrm{e}=1.6 \times 10^{-19} \mathrm{C}$ )
(Given, $\mathrm{h}=6.6 \times 10^{-34} \mathrm{Js}, \mathrm{e}=1.6 \times 10^{-19} \mathrm{C}$ )
18
A transformer has an efficiency of $80 \%$ and works at $10 \mathrm{~V}$ and $4 \mathrm{~kW}$. If the secondary voltage is $240 \mathrm{~V}$, then the current in the secondary coil is :
19
In an ammeter, $5 \%$ of the main current passes through the galvanometer. If resistance of the galvanometer is $\mathrm{G}$, the resistance of ammeter will be :
20
A disc of radius $\mathrm{R}$ and mass $\mathrm{M}$ is rolling horizontally without slipping with speed $v$. It then moves up an inclined smooth surface as shown in figure. The maximum height that the disc can go up the incline is :


21
A mass $m$ is suspended from a spring of negligible mass and the system oscillates with a frequency $f_1$. The frequency of oscillations if a mass $9 \mathrm{~m}$ is suspended from the same spring is $f_2$. The value of $\frac{f_1}{f_2} \mathrm{i}$ ________.
22
In an electrical circuit drawn below the amount of charge stored in the capacitor is _______ $\mu$ C.


23
A particle initially at rest starts moving from reference point $x=0$ along $x$-axis, with velocity $v$ that varies as $v=4 \sqrt{x} \mathrm{~m} / \mathrm{s}$. The acceleration of the particle is __________ $\mathrm{ms}^{-2}$.
24
Suppose a uniformly charged wall provides a uniform electric field of $2 \times 10^4 \mathrm{~N} / \mathrm{C}$ normally. A charged particle of mass $2 \mathrm{~g}$ being suspended through a silk thread of length $20 \mathrm{~cm}$ and remain stayed at a distance of $10 \mathrm{~cm}$ from the wall.
Then the charge on the particle will be $\frac{1}{\sqrt{x}} \mu \mathrm{C}$ where $x=$ ___________ . [use $\mathrm{g}=10 \mathrm{~m} / \mathrm{s}^2$ ]
Then the charge on the particle will be $\frac{1}{\sqrt{x}} \mu \mathrm{C}$ where $x=$ ___________ . [use $\mathrm{g}=10 \mathrm{~m} / \mathrm{s}^2$ ]
25
A moving coil galvanometer has 100 turns and each turn has an area of $2.0 \mathrm{~cm}^2$. The magnetic field produced by the magnet is $0.01 \mathrm{~T}$ and the deflection in the coil is 0.05 radian when a current of $10 \mathrm{~mA}$ is passed through it. The torsional constant of the suspension wire is $x \times 10^{-5} \mathrm{~N}-\mathrm{m} / \mathrm{rad}$. The value of $x$ is _______ .
26
One end of a metal wire is fixed to a ceiling and a load of $2 \mathrm{~kg}$ hangs from the other end. A similar wire is attached to the bottom of the load and another load of $1 \mathrm{~kg}$ hangs from this lower wire. Then the ratio of longitudinal strain of upper wire to that of the lower wire will be ________.
[Area of cross section of wire $=0.005 \mathrm{~cm}^2, \mathrm{Y}=2 \times 10^{11} \mathrm{Nm}^{-2}$ and $\mathrm{g}=10 \mathrm{~ms}^{-2}$ ]
[Area of cross section of wire $=0.005 \mathrm{~cm}^2, \mathrm{Y}=2 \times 10^{11} \mathrm{Nm}^{-2}$ and $\mathrm{g}=10 \mathrm{~ms}^{-2}$ ]
27
In Young's double slit experiment, monochromatic light of wavelength 5000 Å is used. The slits are $1.0 \mathrm{~mm}$ apart and screen is placed at $1.0 \mathrm{~m}$ away from slits. The distance from the centre of the screen where intensity becomes half of the maximum intensity for the first time is _________ $\times 10^{-6}$ $\mathrm{m}$.
28
A coil of 200 turns and area $0.20 \mathrm{~m}^2$ is rotated at half a revolution per second and is placed in uniform magnetic field of $0.01 \mathrm{~T}$ perpendicular to axis of rotation of the coil. The maximum voltage generated in the coil is $\frac{2 \pi}{\beta}$ volt. The value of $\beta$ is _______.
29
A uniform rod $A B$ of mass $2 \mathrm{~kg}$ and length $30 \mathrm{~cm}$ at rest on a smooth horizontal surface. An impulse of force $0.2 \mathrm{~Ns}$ is applied to end B. The time taken by the rod to turn through at right angles will be $\frac{\pi}{x} \mathrm{~s}$, where $x=$ _______ .
30
A particular hydrogen-like ion emits the radiation of frequency $3 \times 10^{15} \mathrm{~Hz}$ when it makes transition from $n=2$ to $n=1$. The frequency of radiation emitted in transition from $n=3$ to $n=1$ is $\frac{x}{9} \times 10^{15} \mathrm{~Hz}$, when $x=$ ________ .