1
JEE Main 2022 (Online) 26th June Morning Shift
Numerical
+4
-1

For complete combustion of methanol
CH3OH(I) + $${3 \over 2}$$O2(g) $$\to$$ CO2(g) + 2H2O(I)
the amount of heat produced as measured by bomb calorimeter is 726 kJ mol$$-$$1 at 27$$^\circ$$C. The enthalpy of combustion for the reaction is $$-$$x kJ mol$$-$$1, where x is ___________. (Nearest integer)
(Given : R = 8.3 JK$$-$$1 mol$$-$$1)
Your input ____
2
JEE Main 2022 (Online) 25th June Morning Shift
Numerical
+4
-1

The standard entropy change for the reaction
4Fe(s) + 3O2(g) $$\to$$ 2Fe2O3(s) is $$-$$550 J K$$-$$1 at 298 K.
[Given : The standard enthalpy change for the reaction is $$-$$165 kJ mol$$-$$1]. The temperature in K at which the reaction attains equilibrium is _____________. (Nearest Integer)
Your input ____
3
JEE Main 2021 (Online) 1st September Evening Shift
Numerical
+4
-1

For the reaction, 2NO2(g) $$\rightleftharpoons$$ N2O4(g), when $$\Delta$$S = $$-$$176.0 JK$$-$$1 and $$\Delta$$H = $$-$$57.8 kJ mol$$-$$1, the magnitude of $$\Delta$$G at 298 K for the reaction is ___________ kJ mol$$-$$1. (Nearest integer)
Your input ____
4
JEE Main 2021 (Online) 27th August Evening Shift
Numerical
+4
-1

Two flasks I and II shown below are connected by a valve of negligible volume.
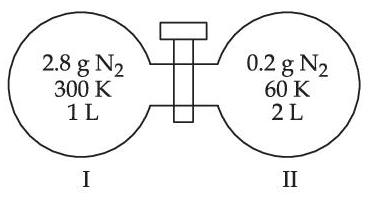
When the valve is opened, the final pressure of the system in bar is x $$\times$$ 10$$-$$2. The value of x is __________. (Integer answer)
[Assume - Ideal gas; 1 bar = 105 Pa; Molar mass of N2 = 28.0 g mol$$-$$1; R = 8.31 J mol$$-$$1 K$$-$$1]

When the valve is opened, the final pressure of the system in bar is x $$\times$$ 10$$-$$2. The value of x is __________. (Integer answer)
[Assume - Ideal gas; 1 bar = 105 Pa; Molar mass of N2 = 28.0 g mol$$-$$1; R = 8.31 J mol$$-$$1 K$$-$$1]
Your input ____
Questions Asked from Thermodynamics (Numerical)
Number in Brackets after Paper Indicates No. of Questions
JEE Main 2025 (Online) 8th April Evening Shift (1)
JEE Main 2025 (Online) 3rd April Evening Shift (2)
JEE Main 2025 (Online) 3rd April Morning Shift (1)
JEE Main 2025 (Online) 28th January Evening Shift (1)
JEE Main 2025 (Online) 28th January Morning Shift (1)
JEE Main 2025 (Online) 24th January Morning Shift (1)
JEE Main 2025 (Online) 23rd January Evening Shift (1)
JEE Main 2025 (Online) 23rd January Morning Shift (1)
JEE Main 2025 (Online) 22nd January Evening Shift (1)
JEE Main 2024 (Online) 9th April Evening Shift (1)
JEE Main 2024 (Online) 9th April Morning Shift (2)
JEE Main 2024 (Online) 8th April Evening Shift (1)
JEE Main 2024 (Online) 8th April Morning Shift (1)
JEE Main 2024 (Online) 6th April Evening Shift (1)
JEE Main 2024 (Online) 6th April Morning Shift (1)
JEE Main 2024 (Online) 5th April Evening Shift (1)
JEE Main 2024 (Online) 5th April Morning Shift (1)
JEE Main 2024 (Online) 4th April Evening Shift (1)
JEE Main 2024 (Online) 4th April Morning Shift (1)
JEE Main 2024 (Online) 1st February Evening Shift (1)
JEE Main 2024 (Online) 31st January Evening Shift (1)
JEE Main 2024 (Online) 31st January Morning Shift (1)
JEE Main 2024 (Online) 30th January Evening Shift (1)
JEE Main 2024 (Online) 30th January Morning Shift (1)
JEE Main 2024 (Online) 29th January Evening Shift (1)
JEE Main 2024 (Online) 27th January Evening Shift (1)
JEE Main 2024 (Online) 27th January Morning Shift (1)
JEE Main 2023 (Online) 15th April Morning Shift (1)
JEE Main 2023 (Online) 13th April Morning Shift (1)
JEE Main 2023 (Online) 12th April Morning Shift (1)
JEE Main 2023 (Online) 11th April Evening Shift (1)
JEE Main 2023 (Online) 11th April Morning Shift (1)
JEE Main 2023 (Online) 10th April Evening Shift (1)
JEE Main 2023 (Online) 8th April Evening Shift (1)
JEE Main 2023 (Online) 8th April Morning Shift (1)
JEE Main 2023 (Online) 6th April Evening Shift (1)
JEE Main 2023 (Online) 6th April Morning Shift (2)
JEE Main 2023 (Online) 1st February Evening Shift (1)
JEE Main 2023 (Online) 1st February Morning Shift (1)
JEE Main 2023 (Online) 31st January Evening Shift (1)
JEE Main 2023 (Online) 31st January Morning Shift (1)
JEE Main 2023 (Online) 30th January Evening Shift (1)
JEE Main 2023 (Online) 30th January Morning Shift (1)
JEE Main 2023 (Online) 25th January Evening Shift (1)
JEE Main 2023 (Online) 25th January Morning Shift (1)
JEE Main 2023 (Online) 24th January Evening Shift (2)
JEE Main 2023 (Online) 24th January Morning Shift (1)
JEE Main 2022 (Online) 29th July Morning Shift (1)
JEE Main 2022 (Online) 28th July Evening Shift (1)
JEE Main 2022 (Online) 27th July Evening Shift (1)
JEE Main 2022 (Online) 27th July Morning Shift (1)
JEE Main 2022 (Online) 26th July Evening Shift (1)
JEE Main 2022 (Online) 26th July Morning Shift (1)
JEE Main 2022 (Online) 25th July Evening Shift (1)
JEE Main 2022 (Online) 25th July Morning Shift (1)
JEE Main 2022 (Online) 30th June Morning Shift (1)
JEE Main 2022 (Online) 29th June Evening Shift (1)
JEE Main 2022 (Online) 29th June Morning Shift (1)
JEE Main 2022 (Online) 28th June Evening Shift (1)
JEE Main 2022 (Online) 28th June Morning Shift (1)
JEE Main 2022 (Online) 27th June Evening Shift (1)
JEE Main 2022 (Online) 26th June Evening Shift (1)
JEE Main 2022 (Online) 26th June Morning Shift (1)
JEE Main 2022 (Online) 25th June Morning Shift (1)
JEE Main 2021 (Online) 1st September Evening Shift (1)
JEE Main 2021 (Online) 27th August Evening Shift (2)
JEE Main 2021 (Online) 27th August Morning Shift (1)
JEE Main 2021 (Online) 26th August Evening Shift (1)
JEE Main 2021 (Online) 26th August Morning Shift (1)
JEE Main 2021 (Online) 27th July Evening Shift (1)
JEE Main 2021 (Online) 27th July Morning Shift (1)
JEE Main 2021 (Online) 25th July Evening Shift (1)
JEE Main 2021 (Online) 25th July Morning Shift (1)
JEE Main 2021 (Online) 22th July Evening Shift (1)
JEE Main 2021 (Online) 20th July Evening Shift (1)
JEE Main 2021 (Online) 18th March Morning Shift (1)
JEE Main 2021 (Online) 17th March Morning Shift (1)
JEE Main 2021 (Online) 16th March Evening Shift (1)
JEE Main 2021 (Online) 26th February Evening Shift (1)
JEE Main 2021 (Online) 26th February Morning Shift (2)
JEE Main 2021 (Online) 25th February Evening Shift (1)
JEE Main 2021 (Online) 25th February Morning Shift (2)
JEE Main 2021 (Online) 24th February Evening Shift (1)
JEE Main 2020 (Online) 5th September Evening Slot (1)
JEE Main 2020 (Online) 2nd September Evening Slot (1)
JEE Main 2020 (Online) 2nd September Morning Slot (1)
JEE Main 2020 (Online) 8th January Evening Slot (1)
JEE Main 2020 (Online) 8th January Morning Slot (1)
JEE Main 2020 (Online) 7th January Evening Slot (1)
JEE Main 2020 (Online) 7th January Morning Slot (1)
JEE Main Subjects
Physics
Mechanics
Units & Measurements Vector Algebra Motion in a Straight Line Motion in a Plane Circular Motion Laws of Motion Work Power & Energy Center of Mass and Collision Rotational Motion Properties of Matter Heat and Thermodynamics Simple Harmonic Motion Waves Gravitation
Electricity
Electrostatics Current Electricity Capacitor Magnetic Effect of Current Magnetic Properties of Matter Electromagnetic Induction Alternating Current Electromagnetic Waves
Optics
Modern Physics
Chemistry
Physical Chemistry
Some Basic Concepts of Chemistry Structure of Atom Redox Reactions Gaseous State Chemical Equilibrium Ionic Equilibrium Solutions Thermodynamics Electrochemistry Chemical Kinetics and Nuclear Chemistry Solid State Surface Chemistry
Inorganic Chemistry
Periodic Table & Periodicity Chemical Bonding & Molecular Structure Isolation of Elements Hydrogen s-Block Elements p-Block Elements d and f Block Elements Coordination Compounds Salt Analysis Environmental Chemistry
Organic Chemistry
Mathematics
Algebra
Sets and Relations Logarithm Quadratic Equation and Inequalities Sequences and Series Mathematical Induction Binomial Theorem Matrices and Determinants Permutations and Combinations Probability Vector Algebra 3D Geometry Complex Numbers Statistics Mathematical Reasoning
Trigonometry
Trigonometric Ratio and Identites Trigonometric Equations Inverse Trigonometric Functions Properties of Triangle Height and Distance
Coordinate Geometry
Calculus