Practical Organic Chemistry · Chemistry · JEE Main
MCQ (Single Correct Answer)
A mixture of 1 g each of chlorobenzene, aniline, and benzoic acid is dissolved in 50 mL ethyl acetate and placed in a separating funnel. 5 M NaOH (30 mL) was added in the same funnel. The funnel was shaken vigorously and then kept aside. The ethyl acetate layer in the funnel contains :
Match List - I with List - II
| List - I (Separation of) |
List - II (Separation Technique) |
||
|---|---|---|---|
| (A) | Aniline from aniline-water mixture | (I) | Simple distillation |
| (B) | Glycerol from spent-lye in soap industry | (II) | Fractional distillation |
| (C) | Different fractions of crude oil in petroleum industry | (III) | Distillation at reduced pressure |
| (D) | Chloroform-Aniline mixture | (IV) | Steam distillation |
Choose the correct answer from the options given below :
A toxic compound " A " when reacted with NaCN in aqueous acidic medium yields an edible cooking component and food preservative " B ". " B " is converted to " C " by diborane and can be used as an additive to petrol to reduce emission. "C" upon reaction with oleum at $140^{\circ} \mathrm{C}$ yields an inhalable anesthetic " D ". Identify " A ", " B ", " C " & " D ", respectively :
$$ \text { Match List - I with List - II. } $$
| List - I (Purification technique) |
List - II (Mixture of organic compounds) |
||
|---|---|---|---|
| (A) | $$ \text { Distillation (simple) } $$ |
(I) | Diesel + Petrol |
| (B) | $$ \text { Fractional distillation } $$ |
(II) | Aniline + Water |
| (C) | $$ \text { Distillation under reduced pressure } $$ |
(III) | Chloroform + Aniline |
| (D) | $$ \text { Steam distillation } $$ |
(IV) | Glycerol + Spent-lye |
$$ \text { Choose the correct answer from the options given below : } $$
Given below are two statements:
Statement I: In Lassaigne's test, the covalent organic molecules are transformed into ionic compounds.
Statement II: The sodium fusion extract of an organic compound having N and S gives prussian blue colour with $\mathrm{FeSO}_4$ and $\mathrm{Na}_4\left[\mathrm{Fe}(\mathrm{CN})_6\right]$
In the light of the above statements, choose the correct answer from the options given below
Which of the following compound can give positive iodoform test when treated with aqueous $$\mathrm{KOH}$$ solution followed by potassium hypoiodite.
Match List I with List II
| LIST I (Test) |
LIST II (Observation) |
||
|---|---|---|---|
| A. | $$\mathrm{Br_2}$$ water test | I. | Yellow orange or orange red precipitate formed |
| B. | Ceric ammonium nitrate test | II. | Reddish orange colour disappears |
| C. | Ferric chloride test | III. | Red colour appears |
| D. | 2, 4 - DNP test | IV. | Blue, Green, Violet or Red colour appear |
Choose the correct answer from the options given below:
Identify the incorrect statements regarding primary standard of titrimetric analysis.
(A) It should be purely available in dry form.
(B) It should not undergo chemical change in air.
(C) It should be hygroscopic and should react with another chemical instantaneously and stoichiometrically.
(D) It should be readily soluble in water.
(E) $$\mathrm{KMnO}_4$$ & $$\mathrm{NaOH}$$ can be used as primary standard.
Choose the correct answer from the options given below :
Match List I with List II
| LIST I (Test) |
LIST II (Identification) |
||
|---|---|---|---|
| A. | Bayer's test | I. | Phenol |
| B. | Ceric ammonium nitrate test | II. | Aldehyde |
| C. | Phthalein dye test | III. | Alcoholic-OH group |
| D. | Schiff's test | IV. | Unsaturation |
Choose the correct answer from the options given below :
The correct statement among the following, for a "chromatography" purification method is :
Which of the following statements are correct?
A. Glycerol is purified by vacuum distillation because it decomposes at its normal boiling point.
B. Aniline can be purified by steam distillation as aniline is miscible in water.
C. Ethanol can be separated from ethanol water mixture by azeotropic distillation because it forms azeotrope.
D. An organic compound is pure, if mixed M.P. is remained same.
Choose the most appropriate answer from the options given below :
Statement (I) : Potassium hydrogen phthalate is a primary standard for standardisation of sodium hydroxide solution.
Statement (II) : In this titration phenolphthalein can be used as indicator.
In the light of the above statements, choose the most appropriate answer from the options given below :
The fragrance of flowers is due to the presence of some steam volatile organic compounds called essential oils. These are generally insoluble in water at room temperature but are miscible with water vapour in vapour phase. A suitable method for the extraction of these oils from the flowers is -
Match List I with List II
| List - I (Technique) | List - II (Application | ||
|---|---|---|---|
| (A) | Distillation | (I) | Separation of glycerol from spent-lye |
| (B) | Fractional distillation | (II) | Aniline - Water mixture |
| (C) | Steam distillation | (III) | Separation of crude oil fractions |
| (D) | Distillation under reduced pressure | (IV) | Chloroform - Aniline |
Choose the correct answer from the options given below:
Chromatographic technique/s based on the principle of differential adsorption is / are
A. Column chromatography
B. Thin layer chromatography
C. Paper chromatography
Choose the most appropriate answer from the options given below:
In chromyl chloride test for confirmation of $$\mathrm{Cl}^{-}$$ ion, a yellow solution is obtained. Acidification of the solution and addition of amyl alcohol and $$10 \% \mathrm{~H}_2 \mathrm{O}_2$$ turns organic layer blue indicating formation of chromium pentoxide. The oxidation state of chromium in that is
The technique used for purification of steam volatile water immiscible substances is :
In Carius tube, an organic compound '$$\mathrm{X}$$' is treated with sodium peroxide to form a mineral acid 'Y'.
The solution of $$\mathrm{BaCl}_{2}$$ is added to '$$\mathrm{Y}$$' to form a precipitate 'Z'. 'Z' is used for the quantitative estimation of an extra element. '$$\mathrm{X}$$' could be
Given below are two statements:
Statement I : Aqueous solution of K$$_2$$Cr$$_2$$O$$_7$$ is preferred as a primary standard in volumetric analysis over Na$$_2$$Cr$$_2$$O$$_7$$ aqueous solution.
Statement II : K$$_2$$Cr$$_2$$O$$_7$$ has a higher solubility in water than Na$$_2$$Cr$$_2$$O$$_7$$.
In the light of the above statements, choose the correct answer from the options given below:
A compound '$$\mathrm{X}$$' when treated with phthalic anhydride in presence of concentrated $$\mathrm{H}_{2} \mathrm{SO}_{4}$$ yields '$$\mathrm{Y}$$'. '$$\mathrm{Y}$$' is used as an acid/base indicator. '$$\mathrm{X}$$' and '$$\mathrm{Y}$$' are respectively
Given below are two statements:
Statement I : Methyl orange is a weak acid.
Statement II : The benzenoid form of methyl orange is more intense/deeply coloured than the quinonoid form.
In the light of the above statement, choose the most appropriate answer from the options given below:
Match List I with List II
| LIST I Element detected |
LIST II Reagent used / Product formed |
||
|---|---|---|---|
| A. | Nitrogen | I. | $$\mathrm{Na_2[Fe(CN)_5NO]}$$ |
| B. | Sulphur | II. | $$\mathrm{AgNO_3}$$ |
| C. | Phosphorous | III. | $$\mathrm{Fe_4[Fe(CN)_6]_3}$$ |
| D. | Halogen | IV. | $$\mathrm{(NH_4)_2MoO_4}$$ |
Choose the correct answer from the options given below:
Given below are two statements :
Statement I : Sulphanilic acid gives esterification test for carboxyl group.
Statement II : Sulphanilic acid gives red colour in Lassigne's test for extra element detection.
In the light of the above statements, choose the most appropriate answer from the options given below :
Compound that will give positive Lassaigne's test for both nitrogen and halogen is :
In base vs. acid titration, at the end point methyl orange is present as
The reagent neutral ferric chloride is used to detect the presence of ______________
The formula of the purple colour formed in Laissaigne's test for sulphur using sodium nitroprusside is :
Given below are two statements :
Statement I : In 'Lassaigne's Test', when both nitrogen and sulphur are present in an organic compound, sodium thiocyanate is formed.
Statement II : If both nitrogen and sulphur are present in an organic compound, then the excess of sodium used in sodium fusion will decompose the sodium thiocyanate formed to give NaCN and Na2S.
In the light of the above statements, choose the most appropriate answer from the options given below :
| List - I Test/Reagents/Observation(s) |
List - II Species detected |
||
|---|---|---|---|
| (a) | Lassaigne's Test | (i) | Carbon |
| (b) | Cu(II) oxide | (ii) | Sulphur |
| (c) | Silver nitrate | (iii) | N, S, P, and halogen |
| (d) | The sodium fusion extract gives black precipitate with acetic acid and lead acetate |
(iv) | Halogen Specifically |
The correct match is :
$$ \begin{array}{|l|l|l|l|} \hline & \begin{array}{l} \text { Molisch's } \\ \text { Test } \end{array} & \begin{array}{l} \text { Barfoed } \\ \text { Test } \end{array} & \begin{array}{l} \text { Biuret } \\ \text { Test } \end{array} \\ \hline \text { A } & \text { Positive } & \text { Negative } & \text { Negative } \\ \hline \text { B } & \text { Positive } & \text { Positive } & \text { Negative } \\ \hline \text { C } & \text { Negative } & \text { Negative } & \text { Positive } \\ \hline \end{array} $$
A, B and C are respectively :
Based on above observation, the element present in the given compound is:
| Test | Inference | |
|---|---|---|
| (a) | Dil. HCl | Insoluble |
| (b) | NaOH solution | soluble |
| (c) | Br2/water | Decolourization |

| Item 'I' (compound) | Item 'II' (reagent) |
||
|---|---|---|---|
| (A) | Lysine | (P) | 1-naphthol |
| (B) | Furfural | (Q) | ninhydrin |
| (C) | Benzylalcohol | (R) | KMnO4 |
| (D) | Styrene | (S) | Ceric ammonium nitrate |
| Test | Inference | |
|---|---|---|
| (a) | 2, 4 - DNP test | Coloured precipitate |
| (b) | Idoform test | Yellow precipitate |
| (c) | Azo-dye test | No dye formation |
Compound 'X' is :
| Item-I (drug) | Item-II (test) | ||
|---|---|---|---|
| A | Chloroxylenol | P | Carbylamine test |
| B | Norethindrone | Q | Sodium hydrogen carbonet test |
| C | Sulphapyridine | R | Ferric chloride test |
| D | Penicillin | S | Bayer's test |
M(Metal ion) + L(Ligand) $$ \to $$ C(Complex) end point is estimated spectrophoto - metrically (through light absorption). If 'M' and 'C' do not absorb light and only 'L' absorbs, then the titration plot between absorbed light (A) versus volume of ligand 'L' (V) would look like :
Numerical
In Dumas' method for estimation of nitrogen 1 g of an organic compound gave 150 mL of nitrogen collected at 300 K temperature and 900 mm Hg pressure. The percentage composition of nitrogen in the compound is _______ % (nearest integer)
(Aqueous tension at $300 \mathrm{~K}=15 \mathrm{~mm} \mathrm{~Hg}$ )
0.1 mol of the following given antiviral compound $(\mathrm{P})$ will weigh ________ $\times 10^{-1} \mathrm{~g}$ (nearest integer).

(Given : molar mass in $\mathrm{g} \mathrm{mol}^{-1} \mathrm{H}: 1, \mathrm{C}: 12, \mathrm{~N}: 14, \mathrm{O}: 16, \mathrm{~F}: 19, \mathrm{I}: 127$ )
In the sulphur estimation, 0.20 g of a pure organic compound gave 0.40 g of barium sulphate. The percentage of sulphur in the compound is __________ $\times 10^{-1} \%$.
(Molar mass : $\mathrm{O}=16, \mathrm{~S}=32, \mathrm{Ba}=137$ in $\mathrm{g} ~\mathrm{mol}^{-1}$ )
In Carius method of estimation of halogen, 0.25 g of an organic compound gave 0.15 g of silver bromide ( AgBr ). The percentage of Bromine in the organic compound is ________ $\times 10^{-1} \%$ (Nearest integer).
(Given : Molar mass of Ag is 108 and Br is $80 \mathrm{~g} \mathrm{~mol}^{-1}$ )

In the given TLC, the distance of spot A & B are 5 cm & 7 cm, from the bottom of TLC plate, respectively.
$$\mathrm{R}_{\mathrm{f}}$$ value of $$\mathrm{B}$$ is $$x \times 10^{-1}$$ times more than $$\mathrm{A}$$. The value of $$x$$ is __________.
On a thin layer chromatographic plate, an organic compound moved by $$3.5 \mathrm{~cm}$$, while the solvent moved by $$5 \mathrm{~cm}$$. The retardation factor of the organic compound is ________ $$\times 10^{-1}$$.
$$0.400 \mathrm{~g}$$ of an organic compound $$(\mathrm{X})$$ gave $$0.376 \mathrm{~g}$$ of $$\mathrm{AgBr}$$ in Carius method for estimation of bromine. $$\%$$ of bromine in the compound $$(\mathrm{X})$$ is ___________.
(Given: Molar mass $$\mathrm{AgBr=188~g~mol^{-1}}$$
$$\mathrm{Br}=80 \mathrm{~g} \mathrm{~mol}^{-1}$$)
$$\mathrm{KMnO}_{4}$$ is titrated with ferrous ammonium sulphate hexahydrate in presence of dilute $$\mathrm{H}_{2} \mathrm{SO}_{4}$$. Number of water molecules produced for 2 molecules of $$\mathrm{KMnO}_{4}$$ is ___________.
In bromination of Propyne, with Bromine, 1, 1, 2, 2-tetrabromopropane is obtained in 27% yield. The amount of 1, 1, 2, 2-tetrabromopropane obtained from 1 g of Bromine in this reaction is ___________ $$\times$$ 10$$-$$1 g. (Nearest integer)
(Molar Mass : Bromine = 80 g/mol)
A sample of $$0.125 \mathrm{~g}$$ of an organic compound when analyzed by Duma's method yields $$22.78 \mathrm{~mL}$$ of nitrogen gas collected over $$\mathrm{KOH}$$ solution at $$280 \mathrm{~K}$$ and $$759 \mathrm{~mm}\, \mathrm{Hg}$$. The percentage of nitrogen in the given organic compound is __________. (Nearest integer)
Given :
(a) The vapour pressure of water of $$280 \mathrm{~K}$$ is $$14.2 \mathrm{~mm} \,\mathrm{Hg}$$.
(b) $$\mathrm{R}=0.082 \mathrm{~L}$$ atm $$\mathrm{K}^{-1} \mathrm{~mol}^{-1}$$

In the above reaction, $$5 \mathrm{~g}$$ of toluene is converted into benzaldehyde with $$92 \%$$ yield. The amount of benzaldehyde produced is ______________ $$\times 10^{-2} \mathrm{~g}$$. (Nearest integer)
While estimating the nitrogen present in an organic compound by Kjeldahl's method, the ammonia evolved from $$0.25 \mathrm{~g}$$ of the compound neutralized $$2.5 \mathrm{~mL}$$ of $$2 \,\mathrm{M} \,\mathrm{H}_{2} \mathrm{SO}_{4}$$. The percentage of nitrogen present in organic compound is ______________.
An organic compound with 51.6% sulfur is heated in a Carius tube. The amount of this compound which will form 0.752 g of barium sulphate is ___________ $$\times$$ 10$$-$$1 g.
(Given molar mass of barium sulphate 233 g mol$$-$$1) (Nearest integer).
Kjeldahl's method was used for the estimation of nitrogen in an organic compound. The ammonia evolved from 0.55 g of the compound neutralised 12.5 mL of 1 M H2SO4 solution. The percentage of nitrogen in the compound is _____________. (Nearest integer)
A 2.0 g sample containing MnO2 is treated with HCl liberating Cl2. The Cl2 gas is passed into a solution of KI and 60.0 mL of 0.1 M Na2S2O3 is required to titrate the liberated iodine. The percentage of MnO2 in the sample is _____________. (Nearest integer)
[Atomic masses (in u) Mn = 55; Cl = 35.5; O = 16, I = 127, Na = 23, K = 39, S = 32]
In the estimation of bromine, 0.5 g of an organic compound gave 0.40 g of silver bromide. The percentage of bromine in the given compound is _________ % (nearest integer)
(Relative atomic masses of Ag and Br are 108u and 80u, respectively).
0.25 g of an organic compound containing chlorine gave 0.40 g of silver chloride in Carius estimation. The percentage of chlorine present in the compound is ___________. [in nearest integer]
(Given : Molar mass of Ag is 108 g mol$$-$$1 and that of Cl is 35.5 g mol$$-$$1)
0.2 g of an organic compound was subjected to estimation of nitrogen by Dumas method in which volume of N2 evolved (at STP) was found to be 22.400 mL. The percentage of nitrogen in the compound is _________. [nearest integer]
(Given : Molar mass of N2 is 28 g mol$$-$$1. Molar volume of N2 at STP : 22.4 L)
$${C_2}{H_7}N + \left( {2x + {y \over 2}} \right)CuO \to xC{O_2} + {y \over 2}{H_2}O + {z \over 2}{N_2} + \left( {2x + {y \over 2}} \right)Cu$$
The value of y is ______________. (Integer answer)
[Atomic mass of K = 39, Mn = 55, O = 16]
(Atomic Mass of Ba = 137 u)
(Atomic masses of Ag and Cl are 107.87 and 35.5 respectively)
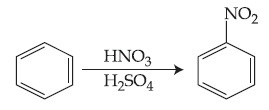
In the above reaction, 3.9 g of benzene on nitration gives 4.92 g of nitrobenzene. The percentage yield of nitrobenzene in the above reaction is ________%. (Round off to the Nearest Integer).
(Given atomic mass : C : 12.0 u, H : 1.0 u, O : 16.0 u, N : 14.0 u)
(i) 4.5 mL
(ii) 4.5 mL
(iii) 4.4 mL
(iv) 4.4 mL
(v) 4.4 mL
If the volume of oxalic acid taken was 10.0 mL then the molarity of the NaOH solution is ________ M. (Rounded off to the nearest integer)
(Atomic mass, Ag = 108, Br = 80 g mol–1)