Some Basic Concepts of Chemistry · Chemistry · JEE Main
MCQ (Single Correct Answer)
On combustion 0.210 g of an organic compound containing C, H and O gave 0.127 g H2O and 0.307 g CO2. The percentages of hydrogen and oxygen in the given organic compound respectively are:
Mass of magnesium required to produce 220 mL of hydrogen gas at STP on reaction with excess of dil. HCl is
Given: Molar mass of Mg is $24 \mathrm{~g} \mathrm{~mol}^{-1}$.
10 mL of 2 M NaOH solution is added to 20 mL of 1 M HCl solution kept in a beaker. Now, 10 mL of this mixture is poured into a volumetric flask of 100 mL containing 2 moles of HCl and made the volume upto the mark with distilled water. The solution in this flask is :
Among $10^{-9} \mathrm{~g}$ (each) of the following elements, which one will have the highest number of atoms?
Element: $\mathrm{Pb}, \mathrm{Po}, \mathrm{Pr}$ and Pt
$$\mathrm{CaCO}_3(\mathrm{~s})+2 \mathrm{HCl}(\mathrm{aq}) \rightarrow \mathrm{CaCl}_2(\mathrm{aq})+\mathrm{CO}_2(\mathrm{~g})+\mathrm{H}_2 \mathrm{O}(\mathrm{l})$$
Consider the above reaction, what mass of $\mathrm{CaCl}_2$ will be formed if 250 mL of 0.76 M HCl reacts with 1000 g of $\mathrm{CaCO}_3$ ?
(Given : Molar mass of $\mathrm{Ca}, \mathrm{C}, \mathrm{O}, \mathrm{H}$ and Cl are $40,12,16,1$ and $35.5 \mathrm{~g} \mathrm{~mol}^{-1}$, respectively)
On complete combustion 1.0 g of an organic compound $(\mathrm{X})$ gave 1.46 g of $\mathrm{CO}_2$ and 0.567 g of $\mathrm{H}_2 \mathrm{O}$. The empirical formula mass of compound $(\mathrm{X})$ is __________ g. (Given molar mass in $\mathrm{g} \mathrm{mol}^{-1} \mathrm{C}: 12, \mathrm{H}: 1, \mathrm{O}: 16$ )
Choose the correct statements.
(A) Weight of a substance is the amount of matter present in it.
(B) Mass is the force exerted by gravity on an object.
(C) Volume is the amount of space occupied by a substance.
(D) Temperatures below 0°C are possible in Celsius scale, but in Kelvin scale negative temperature is not possible.
(E) Precision refers to the closeness of various measurements for the same quantity.
Choose the correct answer from the options given below :
Concentrated nitric acid is labelled as $75 \%$ by mass. The volume in mL of the solution which contains 30 g of nitric acid is ______________.
Given : Density of nitric acid solution is $1.25 \mathrm{~g} / \mathrm{mL}$.
The elemental composition of a compound is $54.2 \% \mathrm{C}, 9.2 \% \mathrm{H}$ and $36.6 \% \mathrm{O}$. If the molar mass of the compound is $132 \mathrm{~g} \mathrm{~mol}^{-1}$, the molecular formula of the compound is : [Given : The relative atomic mass of $\mathrm{C}: \mathrm{H}: \mathrm{O}=12: 1: 16$ ]
$2.8 \times 10^{-3} \mathrm{~mol}$ of $\mathrm{CO}_2$ is left after removing $10^{21}$ molecules from its ' $x$ ' mg sample. The mass of $\mathrm{CO}_2$ taken initially is Given: $\mathrm{N}_{\mathrm{A}}=6.02 \times 10^{23} \mathrm{~mol}^{-1}$
Density of 3 M NaCl solution is $1.25 \mathrm{~g} / \mathrm{mL}$. The molality of the solution is :
Combustion of glucose $$(\mathrm{C}_6 \mathrm{H}_{12} \mathrm{O}_6)$$ produces $$\mathrm{CO}_2$$ and water. The amount of oxygen (in $$\mathrm{g}$$) required for the complete combustion of $$900 \mathrm{~g}$$ of glucose is :
[Molar mass of glucose in $$\mathrm{g} \mathrm{~mol}^{-1}=180$$]
Molality $$(\mathrm{m})$$ of $$3 \mathrm{M}$$ aqueous solution of $$\mathrm{NaCl}$$ is : (Given : Density of solution $$=1.25 \mathrm{~g} \mathrm{~mL}^{-1}$$, Molar mass in $$\mathrm{g} \mathrm{~mol}^{-1}: \mathrm{Na}-23, \mathrm{Cl}-35.5$$)
The density of '$$x$$' $$\mathrm{M}$$ solution ('$$x$$' molar) of $$\mathrm{NaOH}$$ is $$1.12 \mathrm{~g} \mathrm{~mL}^{-1}$$, while in molality, the concentration of the solution is $$3 \mathrm{~m}$$ ( 3 molal). Then $$x$$ is
(Given : Molar mass of $$\mathrm{NaOH}$$ is $$40 \mathrm{~g} / \mathrm{mol}$$)
The number of moles of methane required to produce $$11 \mathrm{~g} \mathrm{~CO}_2(\mathrm{g})$$ after complete combustion is : (Given molar mass of methane in $$\mathrm{g} \mathrm{~mol}^{-1}: 16$$ )
An organic compound has $$42.1 \%$$ carbon, $$6.4 \%$$ hydrogen and remainder is oxygen. If its molecular weight is 342 , then its molecular formula is :
The Molarity (M) of an aqueous solution containing $$5.85 \mathrm{~g}$$ of $$\mathrm{NaCl}$$ in $$500 \mathrm{~mL}$$ water is : (Given : Molar Mass $$\mathrm{Na}: 23$$ and $$\mathrm{Cl}: 35.5 \mathrm{~gmol}^{-1}$$)
A sample of $$\mathrm{CaCO}_3$$ and $$\mathrm{MgCO}_3$$ weighed $$2.21 \mathrm{~g}$$ is ignited to constant weight of $$1.152 \mathrm{~g}$$. The composition of mixture is :
(Given molar mass in $$\mathrm{g} \mathrm{~mol}^{-1} \mathrm{CaCO}_3: 100, \mathrm{MgCO}_3: 84$$)
If a substance '$$A$$' dissolves in solution of a mixture of '$$B$$' and '$$C$$' with their respective number of moles as $$\mathrm{n}_{\mathrm{A}}, \mathrm{n}_{\mathrm{B}}$$ and $$\mathrm{n}_{\mathrm{C}_3}$$. Mole fraction of $$\mathrm{C}$$ in the solution is
The quantity which changes with temperature is :
A metal chloride contains $$55.0 \%$$ of chlorine by weight . $$100 \mathrm{~mL}$$ vapours of the metal chloride at STP weigh $$0.57 \mathrm{~g}$$. The molecular formula of the metal chloride is
(Given: Atomic mass of chlorine is $$35.5 \mathrm{u}$$)
A solution is prepared by adding $$2 \mathrm{~g}$$ of "$$\mathrm{X}$$" to 1 mole of water. Mass percent of "$$\mathrm{X}$$" in the solution is :
Given below are two statements: one is labelled as Assertion $$\mathbf{A}$$ and the other is labelled as Reason $$\mathbf{R}$$
Assertion A : $$3.1500 \mathrm{~g}$$ of hydrated oxalic acid dissolved in water to make $$250.0 \mathrm{~mL}$$ solution will result in $$0.1 \mathrm{~M}$$ oxalic acid solution.
Reason $$\mathbf{R}$$ : Molar mass of hydrated oxalic acid is $$126 \mathrm{~g} \mathrm{~mol}^{-1}$$
In the light of the above statements, choose the correct answer from the options given below.
Match List I with List II
| List - I | List - II ($$\Delta_0$$) | ||
|---|---|---|---|
| A. | 16 g of $$\mathrm{CH_4~(g)}$$ | I. | Weighs 28 g |
| B. | 1 g of $$\mathrm{H_2~(g)}$$ | II. | $$60.2\times10^{23}$$ electrons |
| C. | 1 mole of $$\mathrm{N_2~(g)}$$ | III. | Weighs 32 g |
| D. | 0.5 mol of $$\mathrm{SO_2~(g)}$$ | IV. | Occupies 11.4 L volume of STP |
Choose the correct answer from the options given below:
The number of molecules and moles in 2.8375 litres of O$$_2$$ at STP are respectively
Which of the following have same number of significant figures?
A. 0.00253
B. 1.0003
C. 15.0
D. 163
Choose the correct answer from the options given below
The volume of $$0.02 ~\mathrm{M}$$ aqueous $$\mathrm{HBr}$$ required to neutralize $$10.0 \mathrm{~mL}$$ of $$0.01 ~\mathrm{M}$$ aqueous $$\mathrm{Ba}(\mathrm{OH})_{2}$$ is (Assume complete neutralization)
$1 \mathrm{~L}$ Solution $(\mathrm{X})+\mathrm{AgNO}_{3}$ solution (excess) $\longrightarrow \mathrm{Y}$
$1 \mathrm{~L}$ Solution $(\mathrm{X})+\mathrm{BaCl}_{2}$ solution (excess) $\longrightarrow \mathrm{Z}$
The number of moles of $\mathrm{Y}$ and $\mathrm{Z}$ respectively are
When a hydrocarbon A undergoes combustion in the presence of air, it requires 9.5 equivalents of oxygen and produces 3 equivalents of water. What is the molecular formula of A?
What is the mass ratio of ethylene glycol ($$\mathrm{C_2H_6O_2}$$, molar mass = 62 g/mol) required for making 500 g of 0.25 molal aqueous solution and 250 mL of 0.25 molar aqueous solution?
'25 volume' hydrogen peroxide means
Consider the reaction
$$4 \mathrm{HNO}_{3}(1)+3 \mathrm{KCl}(\mathrm{s}) \rightarrow \mathrm{Cl}_{2}(\mathrm{~g})+\mathrm{NOCl}(\mathrm{g})+2 \mathrm{H}_{2} \mathrm{O}(\mathrm{g})+3 \mathrm{KNO}_{3}(\mathrm{~s})$$
The amount of $$\mathrm{HNO}_{3}$$ required to produce $$110.0 \mathrm{~g}$$ of $$\mathrm{KNO}_{3}$$ is
(Given: Atomic masses of $$\mathrm{H}, \mathrm{O}, \mathrm{N}$$ and $$\mathrm{K}$$ are $$1,16,14$$ and 39, respectively.)
$$ \begin{aligned} &\mathrm{C}(\mathrm{s})+\mathrm{O}_{2}(\mathrm{~g}) \rightarrow \mathrm{CO}_{2}(\mathrm{~g})+400 \mathrm{~kJ} \\ &\mathrm{C}(\mathrm{s})+\frac{1}{2} \mathrm{O}_{2}(\mathrm{~g}) \rightarrow \mathrm{CO}(\mathrm{g})+100 \mathrm{~kJ} \end{aligned} $$
When coal of purity 60% is allowed to burn in presence of insufficient oxygen, 60% of carbon is converted into 'CO' and the remaining is converted into '$$\mathrm{CO}_{2}$$'. The heat generated when $$0.6 \mathrm{~kg}$$ of coal is burnt is _________.
$$ \mathrm{N}_{2(\mathrm{~g})}+3 \mathrm{H}_{2(\mathrm{~g})} \rightleftharpoons 2 \mathrm{NH}_{3(\mathrm{~g})} $$
$$20 \mathrm{~g} \quad ~~~5 \mathrm{~g}$$
Consider the above reaction, the limiting reagent of the reaction and number of moles of $$\mathrm{NH}_{3}$$ formed respectively are :
$$250 \mathrm{~g}$$ solution of $$\mathrm{D}$$-glucose in water contains $$10.8 \%$$ of carbon by weight. The molality of the solution is nearest to
(Given: Atomic Weights are, $$\mathrm{H}, 1 \,\mathrm{u} ; \mathrm{C}, 12 \,\mathrm{u} ; \mathrm{O}, 16 \,\mathrm{u}$$)
In Carius method of estimation of halogen, $$0.45 \mathrm{~g}$$ of an organic compound gave $$0.36 \mathrm{~g}$$ of $$\mathrm{AgBr}$$. Find out the percentage of bromine in the compound.
(Molar masses : $$\mathrm{AgBr}=188 \mathrm{~g} \mathrm{~mol}^{-1} ; \mathrm{Br}=80 \mathrm{~g} \mathrm{~mol}^{-1}$$)
Hemoglobin contains $$0.34 \%$$ of iron by mass. The number of Fe atoms in $$3.3 \mathrm{~g}$$ of hemoglobin is
(Given: Atomic mass of Fe is $$56 \,\mathrm{u}, \mathrm{N}_{\mathrm{A}}=6.022 \times 10^{23} \mathrm{~mol}^{-1}$$.)
$$\mathrm{SO}_{2} \mathrm{Cl}_{2}$$ on reaction with excess of water results into acidic mixture
$$\mathrm{SO}_{2} \mathrm{Cl}_{2}+2 \mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{H}_{2} \mathrm{SO}_{4}+2 \mathrm{HCl}$$
16 moles of $$\mathrm{NaOH}$$ is required for the complete neutralisation of the resultant acidic mixture. The number of moles of $$\mathrm{SO}_{2} \mathrm{Cl}_{2}$$ used is :
Using the rules for significant figures, the correct answer for the expression $${{0.02858 \times 0.112} \over {0.5702}}$$ will be
Production of iron in blast furnace follows the following equation
Fe3O4(s) + 4CO(g) $$\to$$ 3Fe(l) + 4CO2(g)
when 4.640 kg of Fe3O4 and 2.520 kg of CO are allowed to react then the amount of iron (in g) produced is :
[Given : Molar Atomic mass (g mol$$-$$1) : Fe = 56, Molar Atomic mass (g mol$$-$$1) : O = 16, Molar Atomic mass (g mol$$-$$1) : C = 12]
Compound A contains 8.7% Hydrogen, 74% Carbon and 17.3% Nitrogen. The molecular formula of the compound is,
Given : Atomic masses of C, H and N are 12, 1 and 14 amu respectively.
The molar mass of the compound A is 162 g mol$$-$$1.
A commercially sold conc. HCl is 35% HCl by mass. If the density of this commercial acid is 1.46 g/mL, the molarity of this solution is:
(Atomic mass : Cl = 35.5 amu, H = 1 amu)
120 g of an organic compound that contains only carbon and hydrogen gives 330 g of CO2 and 270 g of water on complete combustion. The percentage of carbon and hydrogen, respectively are
If a rocket runs on a fuel (C15H30) and liquid oxygen, the weight of oxygen required and CO2 released for every litre of fuel respectively are :
(Given : density of the fuel is 0.756 g/mL)
(Given atomic mass : Fe = 56, O = 16, Mg = 24, P = 31, C = 12, H = 1)
(molar mass of KI = 166 g mol–1)
N2(g) + 3H2(g) $$ \to $$ 2NH3(g) ;
identify dihydrogen (H2) as a limiting reagent in the following reaction mixtures.
[Molar mass of NaHCO3 = 84 g mol–1]
2C57H110O6(s) + 163 O2(g) $$ \to $$ 114 CO2(g) + 110 H2O(l)
(Atomic wt. of Cl = 35.5 u; Avogadro constant = 6.023 $$ \times $$ 1023 mol-1)
(Given : Atomic wt. - Cr = 52 u, Ba = 137 u)
(Given molar mass of Fe = 56 g mol−1 and molar mass of Cl = 35.5 g mol−1)
(atomic weight of S = 32 amu)
2Al(s) + 6HCl(aq) $$\to$$ 2Al3+ (aq) + 6Cl-(aq) + 3H2(g)
(Avogadro constant, NA = 6.02 $$\times$$ 1023 mol-1)
Numerical
20 mL of sodium iodide solution gave 4.74 g silver iodide when treated with excess of silver nitrate solution. The molarity of the sodium iodide solution is _______ M. (Nearest Integer value)
(Given : Na = 23, I = 127, Ag = 108, N = 14, O = 16 g mol-1)
Butane reacts with oxygen to produce carbon dioxide and water following the equation given below.
$$ \mathrm{C}_4 \mathrm{H}_{10}(\mathrm{~g})+\frac{13}{2} \mathrm{O}_2(\mathrm{~g}) \rightarrow 4 \mathrm{CO}_2(\mathrm{~g})+5 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) $$
If 174.0 kg of butane is mixed with 320.0 kg of $\mathrm{O}_2$, the volume of water formed in liters is
_____________. (Nearest integer)
[Given : (a) Molar mass of C, H, O are $12,1,16 \mathrm{~g} \mathrm{~mol}^{-1}$ respectively, (b) Density of water $\left.=1 \mathrm{~g} \mathrm{~mL}^{-1}\right]$
An organic compound weighing 500 mg , produced 220 mg of $\mathrm{CO}_2$, on complete combustion. The percentage composition of carbon in the compound is _________ $\%$. (nearest integer)
(Given molar mass in $\mathrm{g} \mathrm{mol}^{-1}$ of $\mathrm{C}: 12, \mathrm{O}: 16$ )
Thyroxine, the hormone has given below structure

The percentage of iodine in thyroxine is __________ %. (nearest integer)
(Given molar mass in $\mathrm{g} \mathrm{mol}^{-1} \mathrm{C}: 12, \mathrm{H}: 1, \mathrm{O}: 16, \mathrm{~N}: 14, \mathrm{I}: 127$ )
The amount of calcium oxide produced on heating 150 kg limestone ( $75 \%$ pure) is _________ kg. (Nearest integer)
Given: Molar mass (in $\mathrm{g} \mathrm{mol}^{-1}$ ) of Ca-40, O-16, C-12
Fortification of food with iron is done using $\mathrm{FeSO}_4 \cdot 7 \mathrm{H}_2 \mathrm{O}$. The mass in grams of the $\mathrm{FeSO}_4$. $7 \mathrm{H}_2 \mathrm{O}$ required to achieve 12 ppm of iron in 150 kg of wheat is ______ (Nearest integer)
[Given: Molar mass of $\mathrm{Fe}, \mathrm{S}$ and and O respectively are 56, 32 and $16 \mathrm{~g} \mathrm{~mol}^{-1}$ ]
X g of nitrobenzene on nitration gave 4.2 g of m -dinitrobenzene. X = __________g. (nearest integer)
[Given : molar mass (in $\left.\left.\mathrm{g} \mathrm{mol}^{-1}\right) \mathrm{C}: 12, \mathrm{H}: 1, \mathrm{O}: 16, \mathrm{~N}: 14\right]$
$$ \text {During estimation of nitrogen by Dumas' method of compound } \mathrm{X}(0.42 \mathrm{~g}) $$
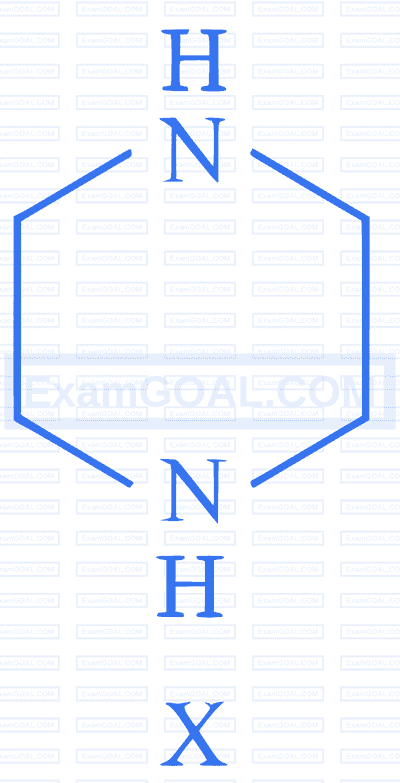
_________mL of $\mathrm{N}_2$ gas will be liberated at STP. (nearest integer)
(Given molar mass in $\mathrm{g}~ \mathrm{mol}^{-1}: \mathrm{C}: 12, \mathrm{H}: 1, \mathrm{~N}: 14$ )0.5 g of an organic compound on combustion gave 1.46 g of $\mathrm{CO}_2$ and 0.9 g of $\mathrm{H}_2 \mathrm{O}$. The percentage of carbon in the compound is _______________. (Nearest integer)
[Given : Molar mass (in $\left.\mathrm{g} \mathrm{mol}^{-1}\right) \mathrm{C}: 12, \mathrm{H}: 1, \mathrm{O}: 16$ ]
The molarity of a $70 \%$ (mass/mass) aqueous solution of a monobasic acid (X) is _________ $\times 10^{-1}$ M (Nearest integer)
[Given: Density of aqueous solution of (X) is $1.25 \mathrm{~g} \mathrm{~mL}^{-1}$
Molar mass of the acid is $70 \mathrm{~g} \mathrm{~mol}^{-1}$ ]
Quantitative analysis of an organic compound (X) shows following % composition.
C : $14.5 \%$
Cl : 64.46%
H: 1.8 %
(Empirical formula mass of the compound $(\mathrm{X})$ is _________ $\times 10^{-1}$
(Given molar mass in $\mathrm{g} \mathrm{~mol}^{-1}$ of $\mathrm{C}: 12, \mathrm{H}: 1, \mathrm{O}: 16, \mathrm{Cl}: 35.5$)
Consider the following reaction occurring in the blast furnace:
$$\mathrm{Fe}_3 \mathrm{O}_{4(\mathrm{~s})}+4 \mathrm{CO}_{(\mathrm{g})} \rightarrow 3 \mathrm{Fe}_{(\mathrm{l})}+4 \mathrm{CO}_{2(\mathrm{~g})}$$
' $x$ ' kg of iron is produced when $2.32 \times 10^3 \mathrm{~kg} \mathrm{Fe}_3 \mathrm{O}_4$ and $2.8 \times 10^2 \mathrm{~kg} \mathrm{CO}$ are brought together in the furnace. The value of ' $x$ ' is _________ . (nearest integer)
{Given: molar mass of $\mathrm{Fe}_3 \mathrm{O}_4=232 \mathrm{~g} \mathrm{~mol}^{-1}$
molar mass of $\mathrm{CO}=28 \mathrm{~g} \mathrm{~mol}^{-1}$
molar mass of $\mathrm{Fe}=56 \mathrm{~g} \mathrm{~mol}^{-1}$}
Xg of benzoic acid on reaction with aq $\mathrm{NaHCO}_3$ released $\mathrm{CO}_2$ that occupied 11.2 L volume at STP.
X is _________ g.
0.01 mole of an organic compound $(X)$ containing $10 \%$ hydrogen, on complete combustion produced $0.9 \mathrm{~g} \mathrm{H}_2 \mathrm{O}$. Molar mass of $(\mathrm{X})$ is _________ $\mathrm{g} \mathrm{~mol}^{-1}$.
When 81.0 g of aluminium is allowed to react with 128.0 g of oxygen gas, the mass of aluminium oxide produced in grams is ________ . (Nearest integer)
Given :
Molar mass of Al is $27.0 \mathrm{~g} \mathrm{~mol}^{-1}$
Molar mass of O is $16.0 \mathrm{~g} \mathrm{~mol}^{-1}$
During " S " estimation, 160 mg of an organic compound gives 466 mg of barium sulphate. The percentage of Sulphur in the given compound is _________ %.
(Given molar mass in $\mathrm{g} \mathrm{~mol}^{-1}$ of $\mathrm{Ba}: 137, \mathrm{~S}: 32, {\mathrm{O}: 16}$)
20 mL of 2 M NaOH solution is added to 400 mL of 0.5 M NaOH solution. The final concentration of the solution is _________ $\times 10^{-2} \mathrm{M}$. (Nearest integer)
Molarity $$(\mathrm{M})$$ of an aqueous solution containing $$x \mathrm{~g}$$ of anhyd. $$\mathrm{CuSO}_4$$ in $$500 \mathrm{~mL}$$ solution at $$32^{\circ} \mathrm{C}$$ is $$2 \times 10^{-1} \mathrm{M}$$. Its molality will be _________ $$\times 10^{-3} \mathrm{~m}$$. (nearest integer). [Given density of the solution $$=1.25 \mathrm{~g} / \mathrm{mL}$$]
A solution is prepared by adding 1 mole ethyl alcohol in 9 mole water. The mass percent of solute in the solution is ________ (Integer answer) (Given : Molar mass in $$\mathrm{g} \mathrm{~mol}^{-1}$$ Ethyl alcohol : 46 water : 18)
Molality of an aqueous solution of urea is $$4.44 \mathrm{~m}$$. Mole fraction of urea in solution is $$x \times 10^{-3}$$, Value of $$x$$ is ________. (Integer answer)
From $$6.55 \mathrm{~g}$$ of aniline, the maximum amount of acetanilide that can be prepared will be ________ $$\times 10^{-1} \mathrm{~g}$$.
$$\mathrm{Xg}$$ of ethylamine is subjected to reaction with $$\mathrm{NaNO}_2 / \mathrm{HCl}$$ followed by water; evolved dinitrogen gas which occupied $$2.24 \mathrm{~L}$$ volume at STP. X is _________ $$\times 10^{-1} \mathrm{~g}$$.
$$ 3 \mathrm{PbCl}_2+2\left(\mathrm{NH}_4\right)_3 \mathrm{PO}_4 \rightarrow \mathrm{Pb}_3\left(\mathrm{PO}_4\right)_2+6 \mathrm{NH}_4 \mathrm{Cl} $$
If $72 ~\mathrm{mmol}$ of $\mathrm{PbCl}_2$ is mixed with $50 ~\mathrm{mmol}$ of $\left(\mathrm{NH}_4\right)_3 \mathrm{PO}_4$, then the amount of $\mathrm{Pb}_3\left(\mathrm{PO}_4\right)_2$ formed is ________ mmol (nearest integer).
The molarity of $$1 \mathrm{~L}$$ orthophosphoric acid $$\left(\mathrm{H}_3 \mathrm{PO}_4\right)$$ having $$70 \%$$ purity by weight (specific gravity $$1.54 \mathrm{~g} \mathrm{~cm}^{-3}$$) is __________ $$\mathrm{M}$$.
(Molar mass of $$\mathrm{H}_3 \mathrm{PO}_4=98 \mathrm{~g} \mathrm{~mol}^{-1}$$)
Number of moles of methane required to produce $$22 \mathrm{~g} \mathrm{~CO}_{2(\mathrm{~g})}$$ after combustion is $$\mathrm{x} \times 10^{-2}$$ moles. The value of $$\mathrm{x}$$ is _________.
Molar mass of the salt from $$\mathrm{NaBr}, \mathrm{NaNO}_3, \mathrm{KI}$$ and $$\mathrm{CaF}_2$$ which does not evolve coloured vapours on heating with concentrated $$\mathrm{H}_2 \mathrm{SO}_4$$ is ________ $$\mathrm{g} \mathrm{~mol}{ }^{-1}$$.
(Molar mass in $$\mathrm{g} \mathrm{~mol}^{-1}: \mathrm{Na}: 23, \mathrm{~N}: 14, \mathrm{~K}: 39, \mathrm{O}: 16, \mathrm{Br}: 80, \mathrm{I}: 127, \mathrm{~F}: 19, \mathrm{Ca}: 40)$$
The mass of sodium acetate $$\left(\mathrm{CH}_3 \mathrm{COONa}\right)$$ required to prepare $$250 \mathrm{~mL}$$ of $$0.35 \mathrm{~M}$$ aqueous solution is ________ g. (Molar mass of $$\mathrm{CH}_3 \mathrm{COONa}$$ is $$82.02 \mathrm{~g} \mathrm{~mol}^{-1}$$)
$$0.05 \mathrm{~cm}$$ thick coating of silver is deposited on a plate of $$0.05 \mathrm{~m}^2$$ area. The number of silver atoms deposited on plate are ________ $$\times 10^{23}$$. (At mass $$\mathrm{Ag}=108, \mathrm{~d}=7.9 \mathrm{~g} \mathrm{~cm}^{-3}$$)
If $$50 \mathrm{~mL}$$ of $$0.5 \mathrm{M}$$ oxalic acid is required to neutralise $$25 \mathrm{~mL}$$ of $$\mathrm{NaOH}$$ solution, the amount of $$\mathrm{NaOH}$$ in $$50 \mathrm{~mL}$$ of given $$\mathrm{NaOH}$$ solution is ______ g.
Molality of 0.8 M H$$_2$$SO$$_4$$ solution (density 1.06 g cm$$^{-3}$$) is ________ $$\times10^{-3}$$ m.
A solution of $$\mathrm{H}_2 \mathrm{SO}_4$$ is $$31.4 \% \mathrm{H}_2 \mathrm{SO}_4$$ by mass and has a density of $$1.25 \mathrm{~g} / \mathrm{mL}$$. The molarity of the $$\mathrm{H}_2 \mathrm{SO}_4$$ solution is _________ $$\mathrm{M}$$ (nearest integer)
[Given molar mass of $$\mathrm{H}_2 \mathrm{SO}_4=98 \mathrm{~g} \mathrm{~mol}^{-1}$$]
$$9.3 \mathrm{~g}$$ of aniline is subjected to reaction with excess of acetic anhydride to prepare acetanilide. The mass of acetanilide produced if the reaction is $$100 \%$$ completed is _________ $$\times 10^{-1} \mathrm{~g}$$.
(Given molar mass in $$\mathrm{g} \mathrm{~mol}^{-1}$$
$$\begin{aligned} & \mathrm{N}: 14, \mathrm{O}: 16, \\ & \mathrm{C}: 12, \mathrm{H}: 1 \text { ) } \end{aligned}$$
Volume of $$3 \mathrm{M} \mathrm{~NaOH}$$ (formula weight $$40 \mathrm{~g} \mathrm{~mol}^{-1}$$ ) which can be prepared from $$84 \mathrm{~g}$$ of $$\mathrm{NaOH}$$ is __________ $$\times 10^{-1} \mathrm{dm}^3$$.
Mass of methane required to produce $$22 \mathrm{~g}$$ of $$\mathrm{CO}_2$$ after complete combustion is _______ g.
(Given Molar mass in g mol-1 $$\mathrm{C}=12.0$$, $$\mathrm{H}=1.0$$, $$\mathrm{O}=16.0)$$
$$1 \mathrm{~g}$$ of a carbonate $$\left(\mathrm{M}_{2} \mathrm{CO}_{3}\right)$$ on treatment with excess $$\mathrm{HCl}$$ produces $$0.01 \mathrm{~mol}$$ of $$\mathrm{CO}_{2}$$. The molar mass of $$\mathrm{M}_{2} \mathrm{CO}_{3}$$ is __________ $$\mathrm{g} ~\mathrm{mol}^{-1}$$. (Nearest integer)
An organic compound gives $$0.220 \mathrm{~g}$$ of $$\mathrm{CO}_{2}$$ and $$0.126 \mathrm{~g}$$ of $$\mathrm{H}_{2} \mathrm{O}$$ on complete combustion. If the $$\%$$ of carbon is 24 then the $$\%$$ of hydrogen is __________ $$\times 10^{-1}$$. ( Nearest integer)
$$20 \mathrm{~mL}$$ of calcium hydroxide was consumed when it was reacted with $$10 \mathrm{~mL}$$ of unknown solution of $$\mathrm{H}_{2} \mathrm{SO}_{4}$$. Also $$20 \mathrm{~mL}$$ standard solution of $$0.5 ~\mathrm{M} ~\mathrm{HCl}$$ containing 2 drops of phenolphthalein was titrated with calcium hydroxide, the mixture showed pink colour when burette displayed the value of $$35.5 \mathrm{~mL}$$ whereas the burette showed $$25.5 \mathrm{~mL}$$ initially. The concentration of $$\mathrm{H}_{2} \mathrm{SO}_{4}$$ is _____________ M. (Nearest integer)
The volume of hydrogen liberated at STP by treating $$2.4 \mathrm{~g}$$ of magnesium with excess of hydrochloric acid is _________ $$\times ~10^{-2} \mathrm{~L}$$
Given : Molar volume of gas is $$22.4 \mathrm{~L}$$ at STP.
Molar mass of magnesium is $$24 \mathrm{~g} \mathrm{~mol}^{-1}$$
A solution of sugar is obtained by mixing $$200 \mathrm{~g}$$ of its $$25 \%$$ solution and $$500 \mathrm{~g}$$ of its $$40 \%$$ solution (both by mass). The mass percentage of the resulting sugar solution is ___________ (Nearest integer)
$$0.5 \mathrm{~g}$$ of an organic compound $$(\mathrm{X})$$ with $$60 \%$$ carbon will produce __________ $$\times 10^{-1} \mathrm{~g}$$ of $$\mathrm{CO}_{2}$$ on complete combustion.
If 5 moles of $$\mathrm{BaCl}_{2}$$ is mixed with 2 moles of $$\mathrm{Na}_{3} \mathrm{PO}_{4}$$, the maximum number of moles of $$\mathrm{Ba}_{3}\left(\mathrm{PO}_{4}\right)_{2}$$ formed is ___________ (Nearest integer)
The molality of a $$10 \%(\mathrm{v} / \mathrm{v})$$ solution of di-bromine solution in $$\mathrm{CCl}_{4}$$ (carbon tetrachloride) is '$$x$$'. $$x=$$ ____________ $$\times 10^{-2} ~\mathrm{M}$$. (Nearest integer)
[Given : molar mass of $$\mathrm{Br}_{2}=160 \mathrm{~g} \mathrm{~mol}^{-1}$$
atomic mass of $$\mathrm{C}=12 \mathrm{~g} \mathrm{~mol}^{-1}$$
atomic mass of $$\mathrm{Cl}=35.5 \mathrm{~g} \mathrm{~mol}^{-1}$$
density of dibromine $$=3.2 \mathrm{~g} \mathrm{~cm}^{-3}$$
density of $$\mathrm{CCl}_{4}=1.6 \mathrm{~g} \mathrm{~cm}^{-3}$$]
The density of $$3 \mathrm{M}$$ solution of $$\mathrm{NaCl}$$ is $$1.0 \mathrm{~g} \mathrm{~mL}^{-1}$$. Molality of the solution is ____________ $$\times 10^{-2} \mathrm{~m}$$. (Nearest integer).
Given: Molar mass of $$\mathrm{Na}$$ and $$\mathrm{Cl}$$ is $$23$$ and $$35.5 \mathrm{~g} \mathrm{~mol}^{-1}$$ respectively.
$2 \mathrm{C}_{(\mathrm{s})}+\mathrm{O}_{2(\mathrm{~g})} \rightarrow 2 \mathrm{CO}(\mathrm{g})$
when $12 \mathrm{~g}$ carbon is burnt in $48 \mathrm{~g}$ of oxygen, the volume of carbon monoxide produced is ___________ $\times 10^{-1} \mathrm{~L}$ at STP [nearest integer]
[Given: Assume $\mathrm{CO}$ as ideal gas, Mass of $\mathrm{C}$ is $12 \mathrm{~g} \mathrm{~mol}^{-1}$, Mass of $\mathrm{O}$ is $16 \mathrm{~g} \mathrm{~mol}^{-1}$ and molar volume of an ideal gas at STP is $22.7 \mathrm{~L} \mathrm{~mol}^{-1}$ ]
In the sample of $\mathrm{M}_{0.83} \mathrm{O}_{1.00}$, the percentage of metal ions existing in $+2$ oxidation state is __________ $\%$. (nearest integer)
Zinc reacts with hydrochloric acid to give hydrogen and zinc chloride. The volume of hydrogen gas produced at STP from the reaction of $$11.5 \mathrm{~g}$$ of zinc with excess $$\mathrm{HCl}$$ is __________ L (Nearest integer)
(Given : Molar mass of $$\mathrm{Zn}$$ is $$65.4 \mathrm{~g} \mathrm{~mol}^{-1}$$ and Molar volume of $$\mathrm{H}_{2}$$ at $$\mathrm{STP}=22.7 \mathrm{~L}$$ )
On complete combustion, $$0.492 \mathrm{~g}$$ of an organic compound gave $$0.792 \mathrm{~g}$$ of $$\mathrm{CO}_{2}$$. The % of carbon in the organic compound is ___________ (Nearest integer)
The strength of 50 volume solution of hydrogen peroxide is ______ $\mathrm{g} / \mathrm{L}$ (Nearest integer).
Given:Molar mass of $\mathrm{H}_{2} \mathrm{O}_{2}$ is $34 \mathrm{~g} \mathrm{~mol}^{-1}$
Molar volume of gas at $\mathrm{STP}=22.7 \mathrm{~L}$
Some amount of dichloromethane $$\left(\mathrm{CH}_{2} \mathrm{Cl}_{2}\right)$$ is added to $$671.141 \mathrm{~mL}$$ of chloroform $$\left(\mathrm{CHCl}_{3}\right)$$ to prepare $$2.6 \times 10^{-3} \mathrm{M}$$ solution of $$\mathrm{CH}_{2} \mathrm{Cl}_{2}(\mathrm{DCM})$$. The concentration of $$\mathrm{DCM}$$ is ___________ ppm (by mass).
Given :
atomic mass : C = 12
H = 1
Cl = 35.5
density of $$\mathrm{CHCl}_{3}=1.49 \mathrm{~g} \mathrm{~cm}^{-3}$$
The volume of HCl, containing 73 g L$$^{-1}$$, required to completely neutralise NaOH obtained by reacting 0.69 g of metallic sodium with water, is __________ mL. (Nearest Integer)
(Given : molar masses of Na, Cl, O, H, are 23, 35.5, 16 and 1 g mol$$^{-1}$$ respectively.)
When 0.01 mol of an organic compound containing 60% carbon was burnt completely, 4.4 g of CO$$_2$$ was produced. The molar mass of compound is _____________ g mol$$^{-1}$$ (Nearest integer).
Number of hydrogen atoms per molecule of a hydrocarbon A having 85.8% carbon is __________
(Given : Molar mass of A = 84 g mol$$^{-1}$$)
In sulphur estimation, 0.471 g of an organic compound gave 1.4439 g of barium sulphate. The percentage of sulphur in the compound is ____________ (Nearest Integer)
(Given : Atomic mass Ba: 137 u, S: 32 u, O: 16 u)
The number of units, which are used to express concentration of solutions from the following is _________
Mass percent, Mole, Mole fraction, Molarity, ppm, Molality
When $$\mathrm{Fe_{0.93}O}$$ is heated in presence of oxygen, it converts to $$\mathrm{Fe_2O_3}$$. The number of correct statement/s from the following is ________
A. The equivalent weight of $$\mathrm{Fe_{0.93}O}$$ is $${{\mathrm{Molecular\,weight}} \over {0.79}}$$
B. The number of moles of Fe$$^{2+}$$ and Fe$$^{3+}$$ in 1 mole of $$\mathrm{Fe_{0.93}O}$$ is 0.79 and 0.14 respectively
C. $$\mathrm{Fe_{0.93}O}$$ is metal deficient with lattice comprising of cubic closed packed arrangement of O$$^{2-}$$ ions
D. The % composition of Fe$$^{2+}$$ and Fe$$^{3+}$$ in $$\mathrm{Fe_{0.93}O}$$ is 85% and 15% respectively
5 g of NaOH was dissolved in deionized water to prepare a 450 mL stock solution. What volume (in mL) of this solution would be required to prepare 500 mL of 0.1 M solution? _____________
Given : Molar Mass of Na, O and H is 23, 16 and 1 g mol$$^{-1}$$ respectively
A 1.84 mg sample of polyhydric alcoholic compound 'X' of molar mass 92.0 g/mol gave 1.344 mL of $$\mathrm{H}_{2}$$ gas at STP. The number of alcoholic hydrogens present in compound 'X' is ________.
2L of 0.2M H2SO4 is reacted with 2L of 0.1M NaOH solution, the molarity of the resulting product Na2SO4 in the solution is _________ millimolar. (Nearest integer)
In the given reaction,
$$X+Y+3 Z \leftrightarrows X YZ_{3}$$
if one mole of each of $$X$$ and $$Y$$ with $$0.05 \mathrm{~mol}$$ of $$Z$$ gives compound $$X Y Z_{3}$$. (Given : Atomic masses of $$X, Y$$ and $$Z$$ are 10, 20 and 30 amu, respectively.) The yield of $$X YZ_{3}$$ is _____________ g. (Nearest integer)
On complete combustion of $$0.492 \mathrm{~g}$$ of an organic compound containing $$\mathrm{C}, \mathrm{H}$$ and $$\mathrm{O}$$, $$0.7938 \mathrm{~g}$$ of $$\mathrm{CO}_{2}$$ and $$0.4428 \mathrm{~g}$$ of $$\mathrm{H}_{2} \mathrm{O}$$ was produced. The % composition of oxygen in the compound is ___________.
$$20 \mathrm{~mL}$$ of $$0.02 \,\mathrm{M} \,\mathrm{K}_{2} \mathrm{Cr}_{2} \mathrm{O}_{7}$$ solution is used for the titration of $$10 \mathrm{~mL}$$ of $$\mathrm{Fe}^{2+}$$ solution in the acidic medium.
The molarity of $$\mathrm{Fe}^{2+}$$ solution is __________ $$\times \,10^{-2}\, \mathrm{M}$$. (Nearest Integer)
A $$100 \mathrm{~mL}$$ solution of $$\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{MgBr}$$ on treatment with methanol produces $$2.24 \mathrm{~mL}$$ of a gas at STP. The weight of gas produced is _____________ mg. [nearest integer]
Chlorophyll extracted from the crushed green leaves was dissolved in water to make $$2 \mathrm{~L}$$ solution of Mg of concentration $$48\, \mathrm{ppm}$$. The number of atoms of $$\mathrm{Mg}$$ in this solution is $$x \times 10^{20}$$ atoms. The value of $$x$$ is ___________. (Nearest Integer)
(Given : Atomic mass of $$\mathrm{Mg}$$ is $$24 \mathrm{~g} \mathrm{~mol}^{-1} ; \mathrm{N}_{\mathrm{A}}=6.02 \times 10^{23} \mathrm{~mol}^{-1}$$ )
When 800 mL of 0.5 M nitric acid is heated in a beaker, its volume is reduced to half and 11.5 g of nitric acid is evaporated. The molarity of the remaining nitric acid solution is x $$\times$$ 10$$-$$2 M. (Nearest integer)
(Molar mass of nitric acid is 63 g mol$$-$$1)
56.0 L of nitrogen gas is mixed with excess hydrogen gas and it is found that 20 L of ammonia gas is produced. The volume of unused nitrogen gas is found to be _________ L.
A sample of 4.5 mg of an unknown monohydric alcohol, R-OH was added to methylmagnesium iodide. A gas is evolved and is collected and its volume measured to be 3.1 mL. The molecular weight of the unknown alcohol is __________ g/mol. [Nearest integer]
Blister copper is produced by reaction of copper oxide with copper sulphide.
2Cu2O + Cu2S $$\to$$ 6Cu + SO2
When 2.86 $$\times$$ 103 g of Cu2O and 4.77 $$\times$$ 103 g of Cu2S are used for reaction, the mass of copper produced is _____________ g. (nearest integer)
(Atomic mass of Cu = 63.5 a.m. u, S = 32.0 a.m. u, O = 16.0 a.m. u)
The complete combustion of 0.492 g of an organic compound containing 'C', 'H' and 'O' gives 0.793 g of CO2 and 0.442 g of H2O. The percentage of oxygen composition in the organic compound is ______________. (nearest integer)
116 g of a substance upon dissociation reaction, yields 7.5 g of hydrogen, 60 g of oxygen and 48.5 g of carbon. Given that the atomic masses of H, O and C are 1, 16 and 12, respectively. The data agrees with how many formulae of the following?
A. CH3COOH, B. HCHO, C. CH3OOCH3, D. CH3CHO
Two elements A and B which form 0.15 moles of A2B and AB3 type compounds. If both A2B and AB3 weigh equally, then the atomic weight of A is _____________ times of atomic weight of B.
CNG is an important transportation fuel. When 100 g CNG is mixed with 208 g oxygen in vehicles, it leads to the formation of CO2 and H2O and produces large quantity of heat during this combustion, then the amount of carbon dioxide, produced in grams is ____________. [nearest integer]
[Assume CNG to be methane]
The moles of methane required to produce 81 g of water after complete combustion is _____________ $$\times$$ 10$$-$$2 mol. [nearest integer]
On complete combustion 0.30 g of an organic compound gave 0.20 g of carbon dioxide and 0.10 g of water. The percentage of carbon in the given organic compound is _____________. (Nearest integer)
A protein 'A' contains 0.30% of glycine (molecular weight 75). The minimum molar mass of the protein 'A' is __________ $$\times$$ 103 g mol$$-$$1 [nearest integer]
The number of N atoms in 681 g of C7H5N3O6 is x $$\times$$ 1021. The value of x is (NA = 6.02 $$\times$$ 1023 mol$$-$$1) (Nearest Integer)
1 L aqueous solution of H2SO4 contains 0.02 m mol H2SO4. 50% of this solution is diluted with deionized water to give 1 L solution (A). In solution (A), 0.01 m mol of H2SO4 are added. Total m mols of H2SO4 in the final solution is ___________ $$\times$$ 103 m mols.
Number of grams of bromine that will completely react with 5.0 g of pent-1-ene is ___________ $$\times$$ 10$$-$$2 g. (Atomic mass of Br = 80 g/mol) [Nearest Integer]
A 0.166 g sample of an organic compound was digested with conc. H2SO4 and then distilled with NaOH. The ammonia gas evolved was passed through 50.0 mL of 0.5 N H2SO4. The used acid required 30.0 mL of 0.25 N NaOH for complete neutralization. The mass percentage of nitrogen in the organic compound is ____________.
[Given : NA = 6.02 $$\times$$ 1023 mol$$-$$1
Atomic mass of Na = 23.0 u]
[Atomic masses Cu : 63.54u, S : 32u, O : 16u, H : 1u]
[Atomic weight : H = 1.008; C = 12.00; O = 16.00]
[Atomic mass : Ag = 108, Br = 80]
[Atomic Masses - Na : 23.0 u, O : 16.0 u, P : 31.0 u]
The above reaction is carried out in a vessel starting with partial pressure PSO2 = 250 m bar, PO2 = 750 m bar and PSO3 = 0 bar. When the reaction is complete, the total pressure in the reaction vessel is _______ m bar. (Round off of the nearest integer).
[Use : Atomic mass : Na : 23.0 u, O : 16.0 u, H : 1.0 u, Density of H2O : 1.0 g cm$$-$$3]
[Given : Atomic mass of C = 12, H = 1, O = 16 u]
(NA = 6.022 $$\times$$ 1023)
(Use : $$\Delta$$CH(glucose) = $$-$$2700 kJ mol$$-$$1)
4.8 mL, 4.9 mL, 5.0 mL, 5.0 mL and 5.0 mL
Based on these readings, and convention of titrimetric estimation the concentration of Na2CO3 solution is ___________ mM.
(Round off to the Nearest Integer).
[Given : Atomic masses : C : 12.0 u, H : 1.0 u, O : 16.0 u ]
(Given : Atomic masses : C : 12.0 u, H : 1.0 u, N : 14.0 u, Br : 80.0 u]
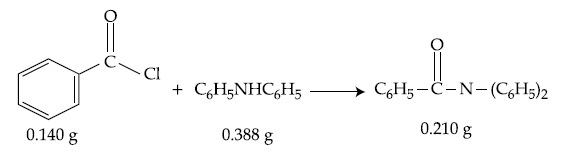
Consider the above reaction. The percentage yield of amide product is __________. (Round off to the Nearest Integer).
(Given : Atomic mass : C : 12.0 u, H : 1.0 u, N : 14.0 u, O : 16.0 u, Cl : 35.5 u)
[Given : Atomic masses : H : 1.0 u, O : 16.0 u ]
If the above equation is balanced with integer coefficients, the value of c is ___________. (Round off to the Nearest Integer).
[Atomic masses : K : 39.0 u; O : 16.0 u; H : 1.0 u]
[Given : Atomic weight in g mol$$-$$1 - Na : 23; N : 14; O : 16]
The value of x is _______. (Rounded off to the nearest integer)
2Fe2+ + H2O2 $$ \to $$ xA + yB
(in basic medium)
2MnO4- + 6H+ + 5H2O2 $$ \to $$ x'C + y'D + z'E
(in acidic medium)
The sum of the stoichiometric coefficients x, y, x', y', and z' for products A, B, C, D and E, respectively, is ______.
(mol. wt. of H2O2 = 34; mol. wt. of KMnO4 = 158)
(molar mass of MgSO4 is 120.37 g/mol)
(Molecular Weight of HNO3 = 63)
M[Co(NH3)6Cl3] = 267.46 g/mol
MAgNO3 = 169.87 g/mol
Atomic weight : Fe = 55.85; S = 32.00; O = 16.00
is 30 m mol L-1 If H2SO4 is used for the flocculatiopn of arsenic sulphide, the amount in grams, of H2SO4 in 250 ml required for the above purposed is ______.
(molecular mass of H2SO4 = 98 g/mol)