Biomolecules · Chemistry · JEE Main
MCQ (Single Correct Answer)
Given below are two statements :
Statement (I) : On hydrolysis, oligo peptides give rise to fewer number of α-amino acids while proteins give rise to a large number of β-amino acids.
Statement (II) : Natural proteins are denatured by acids which convert the water soluble form of fibrous proteins to their water insoluble form.
In the light of the above statements, choose the most appropriate answer from the options given below :
Given below are two statements:
Statement I: D-(+)-glucose + D-(+) fructose $\xrightarrow{-\mathrm{H}_2 \mathrm{O}}$ Sucrose
$$\text { sucrose } \xrightarrow{\text { hydrolysis }} \mathrm{D}-(+) \text { glucose }+\mathrm{D}-(+) \text { fructose }$$
Statement II: Invert sugar is formed during sucrose hydrolysis
In the light of the above statements, choose the correct answer from the options given below
A dipeptide, " $x$ " on complete hydrolysis gives " $y$ " and " $z$ ". " $y$ " on treatment with aq. $\mathrm{HNO}_2$ produces lactic acid. On the other hand " z " on heating gives the following cyclic molecule.

Based on the information given, the dipeptide $X$ is :
Identify the pair of reactants that upon reaction, with elimination of HCl will give rise to the dipeptide Gly-Ala.
Fat soluble vitamins are:
A. Vitamin $\mathrm{B}_1$
B. Vitamin C
C. Vitamin E
D. Vitamin B 12
E. Vitamin K
Choose the correct answer from the options given below:
$$ \text { Which of the following is the correct structure of L-Fructose? } $$
Identify the correct statement among the following :
Identify the essential amino acids from below:
(A) Valine
(B) Proline
(C) Lysine
(D) Threonine
(E) Tyrosine
Choose the correct answer from the options given below:
Match List - I with List - II.
| List - I (Carbohydrate) | List - II (Linkage Source) |
|---|---|
| (A) Amylose | (I) $\beta$ - $C_1 - C_4$, plant |
| (B) Cellulose | (II) $\alpha$ - $C_1 - C_4$, animal |
| (C) Glycogen | (III) $\alpha$ - $C_1 - C_4$, $\alpha$ - $C_1 - C_6$, plant |
| (D) Amylopectin | (IV) $\alpha$ - $C_1 - C_4$, plant |
Choose the correct answer from the options given below :
Match List-I with List-II.
| List - I (Saccharides) | List - II (Glycosidic-linkages found) |
|---|---|
| (A) Sucrose | (I) α 1 $-$ 4 |
| (B) Maltose | (II) α 1 $-$ 4 and α 1 $-$ 6 |
| (C) Lactose | (III) α 1 $-$ β 2 |
| (D) Amylopectin | (IV) β 1 $-$ 4 |
Choose the correct answer from the options given below :
Identify correct conversion during acidic hydrolysis from the following :
(A) starch gives galactose.
(B) cane sugar gives equal amount of glucose and fructose.
(C) milk sugar gives glucose and galactose.
(D) amylopectin gives glucose and fructose.
(E) amylose gives only glucose.
Choose the correct answer from the options given below :
Given below are two statements:
Statement I : D-glucose pentaacetate reacts with 2,4 -dinitrophenylhydrazine
Statement II : Starch, on heating with concentrated sulfuric acid at $100^{\circ} \mathrm{C}$ and $2-3 \mathrm{atmosphere}$ pressure produces glucose.
In the light of the above statements, choose the correct answer from the options given below
Match List - I with List - II.
| List - I | List - II | ||
|---|---|---|---|
| (A) | Adenine | (I) |  |
| (B) | Cytosine | (II) |  |
| (C) | Thymine | (III) |  |
| (D) | Uracil | (IV) |  |
Choose the correct answer from the options given below :
The carbohydrate "Ribose" present in DNA, is
A. A pentose sugar
B. present in pyranose from
C. in "D" configuration
D. a reducing sugar, when free
E. in $\propto$-anomeric form
Choose the correct answer from the options given below:
The $\alpha$-Helix and $\beta$ - Pleated sheet structures of protein are associated with its :
Given below are two statements:
Statement I: Fructose does not contain an aldehydic group but still reduces Tollen's reagent
Statement II: In the presence of base, fructose undergoes rearrangement to give glucose.
In the light of the above statements, choose the correct answer from the options given below
Identify the number of structure/s from the following which can be corelated to D-glyceraldehyde.

Which of the following acids is a vitamin ?
The incorrect statement about Glucose is :
The $$\mathrm{F}^{-}$$ ions make the enamel on teeth much harder by converting hydroxyapatite (the enamel on the surface of teeth) into much harder fluoroapatite having the formula.

The incorrect statement regarding the given structure is
The incorrect statements regarding enzymes are :
(A) Enzymes are biocatalysts.
(B) Enzymes are non-specific and can catalyse different kinds of reactions.
(C) Most Enzymes are globular proteins.
(D) Enzyme - oxidase catalyses the hydrolysis of maltose into glucose.
Choose the correct answer from the option given below :
DNA molecule contains 4 bases whose structure are shown below. One of the structures is not correct, identify the incorrect base structure.
Coagulation of egg, on heating is because of :
Which of the following gives a positive test with ninhydrin ?
Match List I with List II
| LIST I | LIST II | ||
|---|---|---|---|
| A. | $$\alpha$$ - Glucose and $$\alpha$$ - Galactose | I. | Functional isomers |
| B. | $$ \alpha \text { - Glucose and } \beta \text { - Glucose } $$ |
II. | Homologous |
| C. | $$ \alpha \text { - Glucose and } \alpha \text { - Fructose } $$ |
III. | Anomers |
| D. | $$ \alpha \text { - Glucose and } \alpha \text { - Ribose } $$ |
IV. | Epimers |
Choose the correct answer from the options given below:
Which of the following is the correct structure of L-Glucose?
Match List I with List II
| List - I | List - II | ||
|---|---|---|---|
| (A) | Glucose/$$\mathrm{NaHCO_3/\Delta}$$ | (I) | Gluconic acid |
| (B) | $$\text { Glucose } / \mathrm{HNO}_3$$ | (II) | No reaction |
| (C) | $$\text { Glucose } / \mathrm{HI} / \Delta$$ | (III) | n-hexane |
| (D) | Glucose/Bromine water | (IV) | Saccharic acid |
Choose the correct answer from the options given below:
Sugar which does not give reddish brown precipitate with Fehling's reagent, is :
Match List I with List II
| List - I (Bio Polymer) |
List - II (Monomer) |
||
|---|---|---|---|
| (A) | Starch | (I) | nucleotide |
| (B) | Cellulose | (II) | $$\alpha$$-glucose |
| (C) | Nucleic acid | (III) | $$\beta$$-glucose |
| (D) | Protein | (IV) | $$\alpha$$-amino acid |
Choose the correct answer from the options given below:
Type of amino acids obtained by hydrolysis of proteins is :
Which structure of protein remains intact after coagulation of egg white on boiling?
Two nucleotides are joined together by a linkage known as :
The naturally occurring amino acid that contains only one basic functional group in its chemical structure is

The products formed in the above reaction are
L-isomer of tetrose $$\mathrm{X}\left(\mathrm{C}_{4} \mathrm{H}_{8} \mathrm{O}_{4}\right)$$ gives positive Schiff's test and has two chiral carbons. On acetylation, '$$\mathrm{X}$$' yields triacetate. '$$\mathrm{X}$$' also undergoes following reactions

'$$\mathrm{X}$$' is
The one that does not stabilize 2$$^\circ$$ and 3$$^\circ$$ structures of proteins is
Match List I with List II
| LIST I Natural amino acid |
LIST II One letter code |
||
|---|---|---|---|
| A. | Glutamic acid | I. | Q |
| B. | Glutamine | II. | W |
| C. | Tyrosine | III. | E |
| D. | Tryptophan | IV. | Y |
Choose the correct answer from the options given below:
Sulphur (S) containing amino acids from the following are:
(a) isoleucine (b) cysteine (c) lysine (d) methionine (e) glutamic acid
Match List I with List II
| LIST I Natural Amino Acid |
LIST II One Letter Code |
||
|---|---|---|---|
| A. | Arginine | I. | D |
| B. | Aspartic acid | II. | N |
| C. | Asparagine | III. | A |
| D. | Alanine | IV. | R |
Choose the correct answer from the options given below:
Match List I with List II
| LIST I Enzymatic reaction |
LIST II Enzyme |
||
|---|---|---|---|
| A. | Sucrose $$\to$$ Glocuse and Fructose | I. | Zymase |
| B. | Glucose $$\to$$ ethyl alcohol and CO$$_2$$ | II. | Pepsin |
| C. | Starch $$\to$$ Maltose | III. | Invertase |
| D. | Proteins $$\to$$ Amino acids | IV. | Diastase |
Choose the correct answer from the options given below:
All structures given below are of vitamin C. Most stable of them is :
Given below are two statements : one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A) : $$\alpha$$-halocarboxylic acid on reaction with dil. $$\mathrm{NH_3}$$ gives good yield of $$\alpha$$-amino carboxylic acid whereas the yield of amines is very low when prepared from alkyl halides.
Reason (R) : Amino acids exist in zwitter ion form in aqueous medium.
In the light of the above statements, choose the correct answer from the options given below :
The correct representation in six membered pyranose form for the following sugar [X] is

Match List I with List II
| List I | List II | ||
|---|---|---|---|
| Test | Functional group / Class of Compound | ||
| A. | Molisch's Test | I. | Peptide |
| B. | Biuret Test | II. | Carbohydrate |
| C. | Carbylamine Test | III. | Primary amine |
| D. | Schiff's Test | IV. | Aldehyde |
Choose the correct answer from the options given below :
A protein '$$\mathrm{X}$$' with molecular weight of $$70,000 \mathrm{~u}$$, on hydrolysis gives amino acids. One of these amino acid is
Given below are two statements : one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A) : Ketoses give Seliwanoff's test faster than Aldoses.
Reason (R) : Ketoses undergo $$\beta$$-elimination followed by formation of furfural.
In the light of the above statements, choose the correct answer from the options given below :

(F, L, D, Y, I, Q, P are one letter codes for amino acids)
Number of cyclic tripeptides formed with 2 amino acids A and B is :
Match items of Row I with those of Row II.
Row I :

Row II :
(i) $$\alpha$$-$$\mathrm{D}$$-$$(-)$$-$$\mathrm{Fructofuranose}$$
(ii) $$\beta$$-D-$$(-)$$-Fructofuranose
(iii) $$\alpha$$-D-$$(-)$$ Glucopyranose
(iv) $$\beta$$-D-$$(-)$$-Glucopyranose
Correct match is
Given below are two statements. One is labelled as Assertion A and the other is labelled as Reason R.
Assertion A: Amylose is insoluble in water.
Reason R: Amylose is a long linear molecule with more than 200 glucose units.
In the light of the above statements, choose the correct answer from the options given below.
The formulas of A and B for the following reaction sequence

are
For the below given cyclic hemiacetal (X), the correct pyranose structure is :

Match List - I with Match List - II.
| List - I | List - II | ||
|---|---|---|---|
| (A) | Glucose + HI | (I) | Gluconic acid |
| (B) | Glucose + Br$$_2$$ water | (II) | Glucose pentacetate |
| (C) | Glucose + acetic anhydride | (III) | Saccharic acid |
| (D) | Glucose + HNO$$_3$$ | (IV) | Hexane |
Choose the correct answer from the options given below:
A sugar 'X' dehydrates very slowly under acidic condition to give furfural which on further reaction with resorcinol gives the coloured product after sometime. Sugar 'X' is
Animal starch is the other name of
Which one of the following is a reducing sugar?
Glycosidic linkage between C1 of $$\alpha$$-glucose and C2 of $$\beta$$-fructose is found in
During the denaturation of proteins, which of these structures will remain intact?
The sugar produced after complete hydrolysis of DNA is
The structure of protein that is unaffected by heating is
Sugar moiety in DNA and RNA molecules respectively are
When sugar 'X' is boiled with dilute H2SO4 in alcoholic solution, two isomers 'A' and 'B' are formed. 'A' on oxidation with HNO3 yields saccharic acid where as 'B' is laevorotatory. The compound 'X' is :
Stability of $$\alpha$$-Helix structure of proteins depends upon
Given below are two statements.
Statement I : Maltose has two $$\alpha$$-D-glucose units linked at C1 and C4 and is a reducing sugar.
Statement II : Maltose has two monosaccharides : $$\alpha$$-D-glucose and $$\beta$$-D-glucose linked at C1 and C6 and it is a non-reducing sugar.
In the light of the above statements, choose the correct answer from the options given below :
L-isomer of a compound 'A' (C4H8O4) gives a positive test with [Ag(NH3)2]+. Treatment of 'A' with acetic anhydride yields triacetate derivative. Compound 'A' produces an optically active compound (B) and an optically inactive compound (C) on treatment with bromine water and HNO3 respectively. Compound (A) is :
Match List-I with List-II
| List - I Enzyne |
List - II Conversion of |
||
|---|---|---|---|
| A. | Invertase | I. | Starch into maltose |
| B. | Zymase | II. | Maltose into glucose |
| C. | Diastase | III. | Glucose into ethanol |
| D. | Maltase | IV. | Cane sugar into glucose |
Choose the most appropriate answer from the options given below :
Which one of the following is a water soluble vitamin, that is not excreted easily?
A polysaccharide 'X' on boiling with dil H2SO4 at 393 K under 2-3 atm pressure yields 'Y'. 'Y' on treatment with bromine water gives gluconic acid. 'X' contains $$\beta$$-glycosidic linkages only. Compound 'X' is :
Assertion (A) : Sucrose is a disaccharide and a non-reducing sugar.
Reason (R) : Sucrose involves glycosidic linkage between C1 of $$\beta$$-glucose and C2 of $$\alpha$$-fructose.
Choose the most appropriate answer from the options given below :
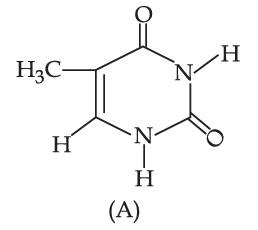
The compound 'A' is a complementary base of _______________ in DNA stands.
$$\mathop {{C_6}{H_{12}}{O_6}}\limits_{Glu\cos e} \buildrel {Enzyme\,B} \over \longrightarrow 2{C_2}{H_5}OH + 2C{O_2}$$
In the above reactions, the enzyme A and enzyme B respectively are :
| List - I | List - II | ||
|---|---|---|---|
| (a) | Sucrose | (i) | $$\beta $$-D-Galactose and $$\beta $$-D-Glucose |
| (b) | Lactose | (ii) | $$\alpha $$-D-Glucose and $$\beta $$-D-Fructose |
| (c) | Maltose | (iii) | $$\alpha $$-D-Glucose and $$\alpha $$-D-Glucose |
Choose the correct answer from the options given below :
| List I | List II | ||
|---|---|---|---|
| (a) | Valium | (i) | Antifertility drug |
| (b) | Morphine | (ii) | Pernicious anaemia |
| (c) | Norethindrone | (iii) | Analgesic |
| (d) | Vitamin $${B_{12}}$$ | (iv) | Tranquilizer |
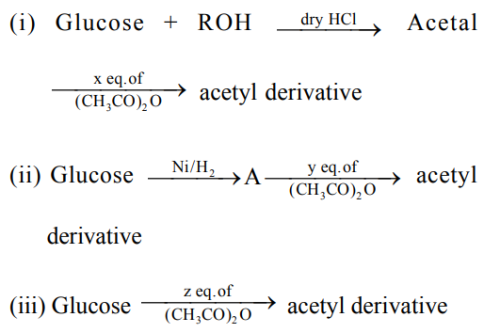
'x', 'y' and 'z' in these reactions are respectively.
(i) Riboflavin (a) Beriberi
(ii) Thiamine (b) Scurvy
(iii) Pyridoxine (c) Cheliosis
(iv) Ascorbic acid (d) Convulsions
I. They activate many enzymes
II. They participate in the oxidation of glucose to produce ATP
III. Along with sodium ions they are responsible for the transmission of nerve signals
| Item I | Item II | ||
|---|---|---|---|
| (A) | Ester test | (P) | Tyr |
| (B) | Carbylamine test | (Q) | Asp |
| (C) | Phthalein dye test |
(R) | Ser |
| (S) | Lys | ||
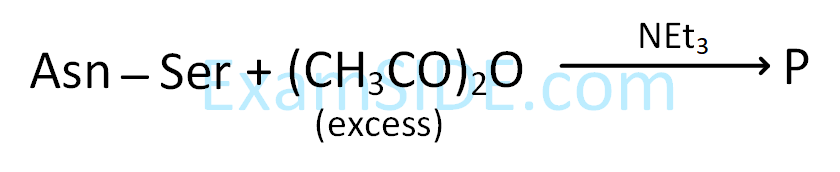

Gly, Asp, Lys, Arg

The pI (isoelectric point) of aspartic acid is :
Numerical
The total number of hydrogen bonds of a DNA-double Helix strand whose one strand has the following sequence of bases is ________.
$$5^{\prime}-\mathrm{G}-\mathrm{G}-\mathrm{C}-\mathrm{A}-\mathrm{A}-\mathrm{A}-\mathrm{T}-\mathrm{C}-\mathrm{G}-\mathrm{G}-\mathrm{C}-\mathrm{T}-\mathrm{A}-3^{\prime}$$
Total number of essential amino acid among the given list of amino acids is ________.
Arginine, Phenylalanine, Aspartic acid, Cysteine, Histidine, Valine, Proline
The total number of carbon atoms present in tyrosine, an amino acid, is ________.
From the vitamins $$\mathrm{A}, \mathrm{B}_1, \mathrm{~B}_6, \mathrm{~B}_{12}, \mathrm{C}, \mathrm{D}, \mathrm{E}$$ and $$\mathrm{K}$$, the number of vitamins that can be stored in our body is _________.
The total number of correct statements, regarding the nucleic acids is _________.
A. RNA is regarded as the reserve of genetic information
B. DNA molecule self-duplicates during cell division
C. DNA synthesizes proteins in the cell
D. The message for the synthesis of particular proteins is present in DNA
E. Identical DNA strands are transferred to daughter cells.
Number of compounds among the following which contain sulphur as heteroatom is ___________.
Furan, Thiophene, Pyridine, Pyrrole, Cysteine, Tyrosine
In an oligopeptide named Alanylglycylphenyl alanyl isoleucine, the number of $$\mathrm{sp}^{2}$$ hybridised carbons is __________.
Number of compounds from the following which will not produce orange red precipitate with Benedict solution is ___________.
Glucose, maltose, sucrose, ribose, 2-deoxyribose, amylose, lactose
Testosterone, which is a steroidal hormone, has the following structure.

The total number of asymmetric carbon atom/s in testosterone is ____________.
Total number of tripeptides possible by mixing of valine and proline is ___________
Uracil is a base present in RNA with the following structure. % of N in uracil is ___________

Given:
Molar mass N = 14 g mol$$^{-1}$$
O = 16 g mol$$^{-1}$$
C = 12 g mol$$^{-1}$$
H = 1 g mol$$^{-1}$$
In a linear tetrapeptide (Constituted with different amino acids), (number of amino acids) $$-$$ (number of peptide bonds) is ________.
C6H12O6 $$\buildrel \text{Zymase} \over \longrightarrow $$ A $$\mathrel{\mathop{\kern0pt\longrightarrow} \limits_\Delta ^\text{NaOI}} $$ B + CHI3
The number of carbon atoms present in the product B is _______________.
The number of oxygens present in a nucleotide formed from a base, that is present only in RNA is ___________.
How many of the given compounds will give a positive Biuret test ____________ ?
Glycine, Glycylalanine, Tripeptide, Biuret
In alanylglycyl leucyl alanyl valine, the number of peptide linkages is ___________.
peptide, Ile-Arg-Pro, is _____.
 groups present in a tripeptide Asp–Glu–Lys is ____.
groups present in a tripeptide Asp–Glu–Lys is ____.