Coordination Compounds · Chemistry · JEE Main
MCQ (Single Correct Answer)
Match the LIST-I with LIST-II
| LIST-I (Complex/ Species) | LIST-II (Shape & magnetic moment) |
|---|---|
| A. [Ni(CO)4] | I. Tetrahedral, 2.8 BM |
| B. [Ni(CN)4]2– | II. Square planar, 0 BM |
| C. [NiCl4]2– | III. Tetrahedral, 0 BM |
| D. [MnBr4]2– | IV. Tetrahedral, 5.9 BM |
Choose the correct answer from the options given below:
The number of species from the following that are involved in sp3d2 hybridization is :
[Co(NH3)6]3+, SF6, [CrF6]3−, [CoF6]3−, [Mn(CN)6]3−, and [MnCl6]3−
Given below are two statements:
Statement I: A homoleptic octahedral complex, formed using monodentate ligands, will not show stereoisomerism.
Statement II: cis- and trans- platin are heteroleptic complexes of Pd.
In the light of the above statements, choose the correct answer from the options given below:
Match List - I with List - II.
| List - I (Complex) | List - II (Primary valency and Secondary valency) |
|---|---|
| (A) [Co(en)2Cl2]Cl | (I) 3, 6 |
| (B) [Pt(NH3)2Cl(NO2)] | (II) 3, 4 |
| (C) Hg [Co(SCN)4] | (III) 2, 6 |
| (D) [Mg (EDTA)]2− | (IV) 2, 4 |
Choose the correct answer from the options given below :
The number of unpaired electrons responsible for the paramagnetic nature of the following complex species are respectively :
[Fe(CN)6]3−, [FeF6]3−, [CoF6]3−, [Mn(CN)6]3−
'X' is the number of acidic oxides among VO2, V2O3, CrO3, V2O5 and Mn2O7. The primary valency of cobalt in
[Co(H2NCH2CH2NH2)3]2(SO4)3 is Y. The value of X + Y is _________.
An octahedral complex having molecular composition $\mathrm{Co} \cdot 5 \mathrm{NH}_3 \cdot \mathrm{Cl}^2 . \mathrm{SO}_4$ has two isomers A and B. The solution of A gives a white precipitate with $\mathrm{AgNO}_3$ solution and the solution of B gives white precipitate with $\mathrm{BaCl}_2$ solution. The type of isomerism exhibited by the complex is,
' $X$ ' is the number of electrons in $t_{2 g}$ orbitals of the most stable complex ion among $\left[\mathrm{Fe}\left(\mathrm{NH}_3\right)_6\right]^{3+},\left[\mathrm{FeCl}_6\right]^{3-}, \quad\left[\mathrm{Fe}\left(\mathrm{C}_2 \mathrm{O}_4\right)_3\right]^{3-}$ and $\left[\mathrm{Fe}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+}$. The nature of oxide of vanadium of the type $\mathrm{V}_2 \mathrm{O}_{\mathrm{X}}$ is :
The correct order of $\left[\mathrm{FeF}_6\right]^{3-},\left[\mathrm{CoF}_6\right]^{3-},\left[\mathrm{Ni}(\mathrm{CO})_4\right]$ and $\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}$ complex species based on the number of unpaired electrons present is:
Which one of the following complexes will have $\Delta_{\mathrm{o}}=0$ and $\mu=5.96$ B.M?
Number of stereoisomers possible for the complexes, $\left[\mathrm{CrCl}_3(\mathrm{py})_3\right]$ and $\left[\mathrm{CrCl}_2(\mathrm{ox})_2\right]^{3-}$ are respectively $(p y=$ pyridine,$o x=$ oxalate $)$
Identify the diamagnetic octahedral complex ions from below ;
A. $\left[\mathrm{Mn}(\mathrm{CN})_6\right]^{3-}$
B. $\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{3+}$
C. $\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{4-}$
D. $\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_3 \mathrm{~F}_3\right]$
Choose the correct answer from the options given below:
$$ \text { Match the LIST-I with LIST-II } $$
| LIST-I (Molecules/ion) |
LIST-II (Hybridisation of central atom) |
||
|---|---|---|---|
| A. | $$ \mathrm{PF}_5 $$ |
I | $$ \mathrm{dsp}^2 $$ |
| B | $$ \mathrm{SF}_6 $$ |
II | $$ \mathrm{sp}^3 \mathrm{~d} $$ |
| C | $$ \mathrm{Ni}(\mathrm{CO})_4 $$ |
III | $$ \mathrm{sp}^3 \mathrm{~d}^2 $$ |
| D | $$ \left[\mathrm{PtCl}_4\right]^{2-} $$ |
IV | $$ \mathrm{sp}^3 $$ |
$$ \text { Choose the correct answer from the options given below: } $$
Given below are two statements :
Statement (I) : In octahedral complexes, when $\Delta_0<\mathrm{P}$ high spin complexes are formed. When $\Delta_0>P$ low spin complexes are formed.
Statement (II) : In tetrahedral complexes because of $\Delta_t < P$, low spin complexes are rarely formed.
In the light of the above statements, choose the most appropriate answer from the options given below :
Identify the homoleptic complexes with odd number of $d$ electrons in the central metal :
(A) $\left[\mathrm{FeO}_4\right]^{2-}$
(B) $\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-}$
(C) $\left[\mathrm{Fe}(\mathrm{CN})_5 \mathrm{NO}\right]^{2-}$
(D) $\left[\mathrm{CoCl}_4\right]^{2-}$
(E) $\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_3 \mathrm{~F}_3\right]$
Choose the correct answer from the options given below :
The calculated spin-only magnetic moments of $K_3[Fe(OH)_6]$ and $K_4[Fe(OH)_6]$ respectively are :
The correct increasing order of stability of the complexes based on $\Delta_0$ value is :
- I. $[\text{Mn}(\text{CN})_6]^{3-}$
- II. $[\text{Co}(\text{CN})_6]^{4-}$
- III. $[\text{Fe}(\text{CN})_6]^{4-}$
- IV. $[\text{Fe}(\text{CN})_6]^{3-}$
Match List - I with List - II.
| List - I (Complex) | List - II (Hybridisation & Magnetic characters) |
|---|---|
| (A) [MnBr4]2- | (I) d2sp3 & diamagnetic |
| (B) [FeF6]3- | (II) sp3d2 & paramagnetic |
| (C) [Co(C2O4)3]3- | (III) sp3 & diamagnetic |
| (D) [Ni(CO)4] | (IV) sp3 & paramagnetic |
Choose the correct answer from the options given below :
Match List - I with List - II.
| List - I (Complex) | List - II (Hybridisation of central metal ion) |
|---|---|
| (A) [CoF6]3- | (I) d2sp3 |
| (B) [NiCl4]2- | (II) sp3 |
| (C) [Co(NH3)6]3+ | (III) sp3d2 |
| (D) [Ni(CN)4]2- | (IV) dsp2 |
The conditions and consequence that favours the $t_{2 \mathrm{~g}}{ }^3 \mathrm{e}_{\mathrm{g}}{ }^1$ configuration in a metal complex are :
When Ethane-1,2-diamine is added progressively to an aqueous solution of Nickel (II) chloride, the sequence of colour change observed will be:
One mole of the octahedral complex compound $\mathrm{Co}\left(\mathrm{NH}_3\right)_5 \mathrm{Cl}_3$ gives 3 moles of ions on dissolution in water. One mole of the same complex reacts with excess of $\mathrm{AgNO}_3$ solution to yield two moles of $\mathrm{AgCl}_{(\mathrm{s})}$. The structure of the complex is:
Identify the coordination complexes in which the central metal ion has $\mathrm{d}^4$ configuration.
(A) $\left[\mathrm{FeO}_4\right]^{2-}$
(B) $\quad\left[\mathrm{Mn}(\mathrm{CN})_6\right]^{3-}$
(C) $\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-}$
(D)  (E) $\left[\mathrm{NiF}_6\right]^{2-}$
(E) $\left[\mathrm{NiF}_6\right]^{2-}$
Choose the correct answer from the options given below :
$\mathrm{CrCl}_3 \cdot \mathrm{xNH}_3$ can exist as a complex. 0.1 molal aqueous solution of this complex shows a depression in freezing point of $0.558^{\circ} \mathrm{C}$. Assuming $100 \%$ ionisation of this complex and coordination number of Cr is 6 , the complex will be (Given $\mathrm{K}_{\mathrm{f}}=1.86 \mathrm{~K} \mathrm{~kg} \mathrm{~mol}^{-1}$ )
The d-electronic configuration of an octahedral Co (II) complex having magnetic moment of 3.95 BM is:
The complex that shows Facial - Meridional isomerism is :
The correct order of the following complexes in terms of their crystal field stabilization energies is :
Identify the homoleptic complex(es) that is/are low spin.
(A) $\left[\mathrm{Fe}(\mathrm{CN})_5 \mathrm{NO}\right]^{2-}$
(B) $\left[\mathrm{CoF}_6\right]^{3-}$
(C) $\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{4-}$
(D) $\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right]^{3+}$
(E) $\left[\mathrm{Cr}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}$
Choose the correct answer from the options given below :
From the magnetic behaviour of $\left[\mathrm{NiCl}_4\right]^{2-}$ (paramagnetic) and $\left[\mathrm{Ni}(\mathrm{CO})_4\right]$ (diamagnetic), choose the correct geometry and oxidation state.
In which of the following complexes the CFSE, $\Delta_o$ will be equal to zero?
Match List I with List II
| LIST I | LIST II | ||
|---|---|---|---|
| A. | $$\mathrm{K}_2\left[\mathrm{Ni}(\mathrm{CN})_4\right]$$ | I. | $$sp^3$$ |
| B. | $$\left[\mathrm{Ni}(\mathrm{CO})_4\right]$$ | II. | $$sp^3d^2$$ |
| C. | $$\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right] \mathrm{Cl}_3$$ | III. | $$dsp^2$$ |
| D. | $$\mathrm{Na}_3\left[\mathrm{CoF}_6\right]$$ | IV. | $$d^2sp^3$$ |
Choose the correct answer from the options given below:
The coordination environment of $$\mathrm{Ca}^{2+}$$ ion in its complex with $$\mathrm{EDTA}^{4-}$$ is :
Given below are two statements : one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A): The total number of geometrical isomers shown by $$[\mathrm{Co}(\mathrm{en})_2 \mathrm{Cl}_2]^{+}$$ complex ion is three.
Reason (R): $$[\mathrm{Co}(\mathrm{en})_2 \mathrm{Cl}_2]^{+}$$ complex ion has an octahedral geometry.
In the light of the above statements, choose the most appropriate answer from the options given below :
Match List I with List II
| LIST I (Complex ion) |
LIST II (Spin only magnetic moment in B.M.) |
||
|---|---|---|---|
| A. | $$ \left[\mathrm{Cr}\left(\mathrm{NH}_3\right)_6\right]^{3+} $$ |
I. | 4.90 |
| B. | $$ \left[\mathrm{NiCl}_4\right]^{2-} $$ |
II. | 3.87 |
| C. | $$ \left[\mathrm{CoF}_6\right]^{3-} $$ |
III. | 0.0 |
| D. | $$ \left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-} $$ |
IV. | 2.83 |
Choose the correct answer from the options given below :
Number of Complexes with even number of electrons in $$\mathrm{t_{2 g}}$$ orbitals is -
$$\left[\mathrm{Fe}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+},\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+},\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+},\left[\mathrm{Cu}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+},\left[\mathrm{Cr}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}$$
An octahedral complex with the formula $$\mathrm{CoCl}_3 \cdot \mathrm{nNH}_3$$ upon reaction with excess of $$\mathrm{AgNO}_3$$ solution gives 2 moles of $$\mathrm{AgCl}$$. Consider the oxidation state of $$\mathrm{Co}$$ in the complex is '$$x$$'. The value of "$$x+n$$" is __________.
Given below are two statements:
Statement I: $$\mathrm{N}\left(\mathrm{CH}_3\right)_3$$ and $$\mathrm{P}\left(\mathrm{CH}_3\right)_3$$ can act as ligands to form transition metal complexes.
Statement II: As N and P are from same group, the nature of bonding of $$\mathrm{N}\left(\mathrm{CH}_3\right)_3$$ and $$\mathrm{P}\left(\mathrm{CH}_3\right)_3$$ is always same with transition metals.
In the light of the above statements, choose the most appropriate answer from the options given below:
Match List I with List II
| LIST I (Compound) |
LIST II (Colour] |
||
|---|---|---|---|
| A. | $$\mathrm{Fe}_4\left[\mathrm{Fe}(\mathrm{CN})_6\right]_3 \cdot \mathrm{xH_2O}$$ | I. | Violet |
| B. | $$\left[\mathrm{Fe}(\mathrm{CN})_5 \mathrm{NOS}\right]^{4-}$$ | II. | Blood Red |
| C. | $$[\mathrm{Fe}(\mathrm{SCN})]^{2+}$$ | III. | Prussian Blue |
| D. | $$\left(\mathrm{NH}_4\right)_3 \mathrm{PO}_4\cdot12 \mathrm{MoO}_3$$ | IV. | Yellow |
Choose the correct answer from the options given below:
Given below are two statements :
Statement I : $$\mathrm{PF}_5$$ and $$\mathrm{BrF}_5$$ both exhibit $$\mathrm{sp}^3 \mathrm{~d}$$ hybridisation.
Statement II : Both $$\mathrm{SF}_6$$ and $$[\mathrm{Co}(\mathrm{NH}_3)_6]^{3+}$$ exhibit $$\mathrm{sp}^3 \mathrm{~d}^2$$ hybridisation.
In the light of the above statements, choose the correct answer from the options given below :
Match List I with List II.
| LIST I Tetrahedral Complex |
LIST II Electronic configuration |
||
|---|---|---|---|
| A. | $$ \mathrm{TiCl}_4 $$ |
I. | $$ \mathrm{e}^2, \mathrm{t}_2^0 $$ |
| B. | $$ \left[\mathrm{FeO}_4\right]^{2-} $$ |
II. | $$ \mathrm{e^4, t_2^3} $$ |
| C. | $$ \left[\mathrm{FeCl}_4\right]^{-} $$ |
III. | $$ \mathrm{e}^0, \mathrm{t}_2^0 $$ |
| D. | $$ \left[\mathrm{CoCl}_4\right]^{2-} $$ |
IV. | $$ \mathrm{e}^2, \mathrm{t}_2^3 $$ |
Choose the correct answer from the options given below :
The correct IUPAC name of $$[\mathrm{PtBr}_2(\mathrm{PMe}_3)_2]$$ is :
Consider the following complexes
(A) $$\left[\mathrm{CoCl}\left(\mathrm{NH}_3\right)_5\right]^{2+}$$, (B) $$\left[\mathrm{Co}(\mathrm{CN})_6\right]^{3-}$$, (C) $$ \left[\mathrm{Co}\left(\mathrm{NH}_3\right)_5\left(\mathrm{H}_2 \mathrm{O}\right)\right]^{3+} $$, (D) $$\left[\mathrm{Cu}\left(\mathrm{H}_2 \mathrm{O}\right)_4\right]^{2+}$$
The correct order of A, B, C and D in terms of wavenumber of light absorbed is :
Match List I with List II
| LIST I (Hybridization) |
LIST II (Orientation in Shape) |
||
|---|---|---|---|
| A. | sp$$^3$$ | I. | Trigonal bipyramidal |
| B. | dsp$$^2$$ | II. | Octahedral |
| C. | sp$$^3$$d | III. | Tetrahedral |
| D. | sp$$^3$$d$$^2$$ | IV. | Square planar |
Choose the correct answer from the options given below:
The number of complexes from the following with no electrons in the $$t_2$$ orbital is ______.
$$\mathrm{TiCl}_4,\left[\mathrm{MnO}_4\right]^{-},\left[\mathrm{FeO}_4\right]^{2-},\left[\mathrm{FeCl}_4\right]^{-},\left[\mathrm{CoCl}_4\right]^{2-}$$
The metal atom present in the complex MABXL (where A, B, X and L are unidentate ligands and $$\mathrm{M}$$ is metal) involves $$\mathrm{sp}^3$$ hybridization. The number of geometrical isomers exhibited by the complex is :
The correct order of ligands arranged in increasing field strength.
Which one of the following complexes will exhibit the least paramagnetic behaviour ? [Atomic number, $$\mathrm{Cr}=24, \mathrm{Mn}=25, \mathrm{Fe}=26, \mathrm{Co}=27$$]
If an iron (III) complex with the formula $$\left[\mathrm{Fe}\left(\mathrm{NH}_3\right)_x(\mathrm{CN})_y\right]^-$$ has no electron in its $$e_g$$ orbital, then the value of $$x+y$$ is
The number of unpaired d-electrons in $$\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+}$$ is ________.
A first row transition metal in its +2 oxidation state has a spin-only magnetic moment value of $$3.86 \mathrm{~BM}$$. The atomic number of the metal is
Number of complexes from the following with even number of unpaired "$$\mathrm{d}$$" electrons is ________ $$[\mathrm{V}(\mathrm{H}_2 \mathrm{O})_6]^{3+},[\mathrm{Cr}(\mathrm{H}_2 \mathrm{O})_6]^{2+},[\mathrm{Fe}(\mathrm{H}_2 \mathrm{O})_6]^{3+},[\mathrm{Ni}(\mathrm{H}_2 \mathrm{O})_6]^{3+},[\mathrm{Cu}(\mathrm{H}_2 \mathrm{O})_6]^{2+}$$ [Given atomic numbers: $$\mathrm{V}=23, \mathrm{Cr}=24, \mathrm{Fe}=26, \mathrm{Ni}=28 \mathrm{Cu}=29$$]
The correct sequence of ligands in the order of decreasing field strength is :
Statement (I) : Dimethyl glyoxime forms a six-membered covalent chelate when treated with $\mathrm{NiCl}_2$ solution in presence of $\mathrm{NH}_4 \mathrm{OH}$.
Statement (II) : Prussian blue precipitate contains iron both in $(+2)$ and $(+3)$ oxidation states.
In the light of the above statements, choose the most appropriate answer from the options given below :
Statement (I) : A solution of $\left[\mathrm{Ni}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}$ is green in colour.
Statement (II) : A solution of $\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}$ is colourless.
In the light of the above statements, choose the most appropriate answer from the options given below :
Select the option with correct property -
Match List I with List II
| List - I (Complex ion) |
List - II (Electronic Configuration) |
||
|---|---|---|---|
| (A) | $$\mathrm{[Cr(H_2O)_6]^{3+}}$$ | (I) | $$t_{2 g}{ }^2 e_g^0$$ |
| (B) | $$\left[\mathrm{Fe}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+}$$ | (II) | $$t_{2 g}{ }^3 e_g{ }^0$$ |
| (C) | $$\left[\mathrm{Ni}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}$$ | (III) | $$t_{2 g}{ }^3 e_g{ }^2$$ |
| (D) | $$\left[\mathrm{V}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+}$$ | (IV) | $$t_{2 g}{ }^6 e_g^2$$ |
Choose the correct answer from the options given below:
The correct statements from following are:
A. The strength of anionic ligands can be explained by crystal field theory.
B. Valence bond theory does not give a quantitative interpretation of kinetic stability of coordination compounds.
C. The hybridization involved in formation of $$\left[\mathrm{Ni}(\mathrm{CN})_4\right]^{2-}$$ complex is $$\mathrm{dsp}^2$$.
D. The number of possible isomer(s) of cis- $$\left[\mathrm{PtCl}_2(\mathrm{en})_2\right]^{2+}$$ is one
Choose the correct answer from the options given below:
The coordination geometry around the manganese in decacarbonyldimanganese $$(0)$$ is
Choose the correct statements from the following :
(A) Ethane-1, 2-diamine is a chelating ligand.
(B) Metallic aluminium is produced by electrolysis of aluminium oxide in presence of cryolite.
(C) Cyanide ion is used as ligand for leaching of silver.
(D) Phosphine act as a ligand in Wilkinson catalyst.
(E) The stability constants of $$\mathrm{Ca}^{2+}$$ and $$\mathrm{Mg}^{2+}$$ are similar with EDTA complexes.
Choose the correct answer from the options given below :
A reagent which gives brilliant red precipitate with Nickel ions in basic medium is
Match List I with List II
| List - I (Substances) |
List - II (Element Present) |
||
|---|---|---|---|
| (A) | Ziegler catalyst | (I) | Rhodium |
| (B) | Blood Pigment | (II) | Cobalt |
| (C) | Wilkinson catalyst | (III) | Iron |
| (D) | Vitamin $$\mathrm{B_{12}}$$ | (IV) | Titanium |
Choose the correct answer from the options given below:
In which one of the following metal carbonyls, $$\mathrm{CO}$$ forms a bridge between metal atoms?
Identity the incorrect pair from the following :
Identify from the following species in which $$\mathrm{d}^2 \mathrm{sp}^3$$ hybridization is shown by central atom :
Consider the following complex ions
$$\begin{aligned} & \mathrm{P}=\left[\mathrm{FeF}_6\right]^{3-} \\ & \mathrm{Q}=\left[\mathrm{V}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+} \\ & \mathrm{R}=\left[\mathrm{Fe}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+} \end{aligned}$$
The correct order of the complex ions, according to their spin only magnetic moment values (in B.M.) is :
Yellow compound of lead chromate gets dissolved on treatment with hot $$\mathrm{NaOH}$$ solution. The product of lead formed is a :
The total number of stereoisomers for the complex $$\left[\mathrm{Cr}(o x)_{2} \mathrm{ClBr}\right]^{3-}$$ (where $$o x=$$ oxalate) is :
Which of the following complexes will exhibit maximum attraction to an applied magnetic field?
The mismatched combinations are
A. Chlorophyll - Co
B. Water hardness - EDTA
C. Photography $$-\left[\mathrm{Ag}(\mathrm{CN})_{2}\right]^{-}$$
D. Wilkinson catalyst $$-\left[\left(\mathrm{Ph}_{3} \mathrm{P}\right)_{3} \mathrm{RhCl}\right]$$
E. Chelating ligand - D-Penicillamine
Choose the correct answer from the options given below :
Match List I with List II
| LIST I Complex |
LIST II CFSE ($$\Delta_0$$) |
||
|---|---|---|---|
| A. | $$\mathrm{[Cu(NH_3)_6]^{2+}}$$ | I. | $$-0.6$$ |
| B. | $$\mathrm{[Ti(H_2O)_6]^{3+}}$$ | II. | $$-2.0$$ |
| C. | $$\mathrm{[Fe(CN)_6]^{3-}}$$ | III. | $$-1.2$$ |
| D. | $$\mathrm{[NiF_6]^{4-}}$$ | IV. | $$-0.4$$ |
Choose the correct answer from the options given below:
Given below are two statements, one is labelled as Assertion A and the other is labelled as Reason R.
Assertion A : $$\left[\mathrm{CoCl}\left(\mathrm{NH}_{3}\right)_{5}\right]^{2+}$$ absorbs at lower wavelength of light with respect to $$\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{5}\left(\mathrm{H}_{2} \mathrm{O}\right)\right]^{3+}$$
Reason R : It is because the wavelength of the light absorbed depends on the oxidation state of the metal ion.
In the light of the above statements, choose the correct answer from the options given below:
If $$\mathrm{Ni}^{2+}$$ is replaced by $$\mathrm{Pt}^{2+}$$ in the complex $$\left[\mathrm{NiCl}_{2} \mathrm{Br}_{2}\right]^{2-}$$, which of the following properties are expected to get changed ?
A. Geometry
B. Geometrical isomerism
C. Optical isomerism
D. Magnetic properties
Match List I with List II
| LIST I Complex |
LIST II Colour |
||
|---|---|---|---|
| A. | $$Mg(N{H_4})P{O_4}$$ | I. | brown |
| B. | $${K_3}[Co{(N{O_2})_6}]$$ | II. | white |
| C. | $$MnO{(OH)_2}$$ | III. | yellow |
| D. | $$F{e_4}{[Fe{(CN)_6}]_3}$$ | IV. | blue |
Choose the correct answer from the options given below :
The magnetic moment is measured in Bohr Magneton (BM).
Spin only magnetic moment of $$\mathrm{Fe}$$ in $$\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{3+}$$ and $$\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}$$ complexes respectively is :
The complex that dissolves in water is :
The set which does not have ambidentate ligand(s) is :
Which of the following complex has a possibility to exist as meridional isomer?
The correct order of the number of unpaired electrons in the given complexes is
A. $$\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}$$
B. $$\left[\mathrm{Fe} \mathrm{F}_{6}\right]^{3-}$$
C. $$\left[\mathrm{CoF}_{6}\right]^{3-}$$
D. $$\left.[\mathrm{Cr} \text { (oxalate})_{3}\right]^{3-}$$
E. $$\left[\mathrm{Ni}(\mathrm{CO})_{4}\right]$$
Choose the correct answer from the options given below:
Match List I with List II
| List - I Complex |
List - II Crystal Field splitting energy ($$\Delta_0$$) |
||
|---|---|---|---|
| A. | $${[Ti{({H_2}O)_6}]^{2 + }}$$ | I. | $$-1.2$$ |
| B. | $${[V{({H_2}O)_6}]^{2 + }}$$ | II. | $$-0.6$$ |
| C. | $${[Mn{({H_2}O)_6}]^{3 + }}$$ | III. | 0 |
| D. | $${[Fe{({H_2}O)_6}]^{3 + }}$$ | IV. | $$-0.8$$ |
Choose the correct answer from the options given below:
The octahedral diamagnetic low spin complex among the following is :
Match List I with List II
| LIST I Coordination Complex |
LIST II Number of unpaired electrons |
||
|---|---|---|---|
| A. | $$\left[\mathrm{Cr}(\mathrm{CN})_{6}\right]^{3-}$$ | I. | 0 |
| B. | $$\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}$$ | II. | 3 |
| C. | $$\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}$$ | III. | 2 |
| D. | $$\left[\mathrm{Ni}\left(\mathrm{NH}_{3}\right)_{6}\right]^{2+}$$ | IV. | 4 |
Choose the correct answer from the options given below:
Which of the following complex is octahedral, diamagnetic and the most stable?
The correct order of spin only magnetic moments for the following complex ions is
The IUPAC name of $$\mathrm{K}_{3}\left[\mathrm{Co}\left(\mathrm{C}_{2} \mathrm{O}_{4}\right)_{3}\right]$$ is:-
Given below are two statements, one is labelled as Assertion $$\mathbf{A}$$ and the other is labelled as Reason $$\mathbf{R}$$.
Assertion A: The spin only magnetic moment value for $$\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}$$ is $$1.74 \mathrm{BM}$$, whereas for $$\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{3+}$$ is $$5.92 \mathrm{BM}$$.
Reason $$\mathbf{R}$$ : In both complexes, $$\mathrm{Fe}$$ is present in +3 oxidation state.
In the light of the above statements, choose the correct answer from the options given below:
The complex cation which has two isomers is :
Which of the following complex will show largest splitting of d-orbitals?
Which of the following are the example of double salt?
A. $$\mathrm{FeSO}_{4} \cdot\left(\mathrm{NH}_{4}\right)_{2} \mathrm{SO}_{4} \cdot 6 \mathrm{H}_{2} \mathrm{O}$$
B. $$\mathrm{CuSO}_{4}\cdot 4 \mathrm{NH}_{3} \cdot \mathrm{H}_{2} \mathrm{O}$$
C. $$\mathrm{K}_{2} \mathrm{SO}_{4} \cdot \mathrm{Al}_{2}\left(\mathrm{SO}_{4}\right)_{3} \cdot 24 \mathrm{H}_{2} \mathrm{O}$$
D. $$\mathrm{Fe}(\mathrm{CN})_{2}\cdot4 \mathrm{KCN}$$
Choose the correct answer :
A solution of $$\mathrm{FeCl_3}$$ when treated with $$\mathrm{K_4[Fe(CN)_6]}$$ gives a prussium blue precipitate due to the formation of :
Cobalt chloride when dissolved in water forms pink colored complex $$\underline{\mathrm{X}}$$ which has octahedral geometry. This solution on treating with conc $$\mathrm{HCl}$$ forms deep blue complex, $$\underline{\mathrm{Y}}$$ which has a $$\underline{\mathrm{Z}}$$ geometry. $$\mathrm{X}, \mathrm{Y}$$ and $$\mathrm{Z}$$, respectively, are
Match List I with List II:
| List I (Complexes) | List II (Hybridisation) | ||
|---|---|---|---|
| A. | $\left[\mathrm{Ni}(\mathrm{CO})_{4}\right]$ | I. | $\mathrm{sp}^{3}$ |
| B. | $\left[\mathrm{Cu}\left(\mathrm{NH}_{3}\right)_{4}\right]^{2+}$ | II. | dsp$^{2}$ |
| C. | $\left[\mathrm{Fe}\left(\mathrm{NH}_{3}\right)_{6}\right]^{2+}$ | III. | $\mathrm{sp}^{3}\mathrm{d}^{2}$ |
| D. | $\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}$ | IV. | $\mathrm{d}^{2} \mathrm{sp}^{3}$ |
To inhibit the growth of tumours, identify the compounds used from the following :
A. EDTA
B. Coordination Compounds of Pt
C. D - Penicillamine
D. Cis - Platin
Choose the correct answer from the option given below :
Which of the following is correct order of ligand field strength?
Correct order of spin only magnetic moment of the following complex ions is :
(Given At.no. Fe : 26, Co : 27)
Chiral complex from the following is :
Here en = ethylene diamine
Match List I with List II
| List I Coordination entity |
List II Wavelength of light absorbed in nm |
||
|---|---|---|---|
| A. | $$\mathrm{[CoCl(NH_3)_5]^{2+}}$$ | I. | 310 |
| B. | $$\mathrm{[Co(NH_3)_6]^{3+}}$$ | II. | 475 |
| C. | $$\mathrm{[Co(CN)_6]^{3-}}$$ | III. | 535 |
| D. | $$\mathrm{[Cu(H_2O)_4]^{2+}}$$ | IV. | 600 |
Choose the correct answer from the options given below :
The hybridization and magnetic behaviour of cobalt ion in $$\mathrm{[Co(NH_3)_6]^{3+}}$$ complex, respectively is :
Which of the following cannot be explained by crystal field theory?
The primary and secondary valencies of cobalt respectively in $$\mathrm{[Co(NH_3)_5Cl]Cl_2}$$ are :
Octahedral complexes of copper(II) undergo structural distortion (Jahn-Teller). Which one of the given copper (II) complexes will show the maximum structural distortion? (en - ethylenediamine; $$\mathrm{H}_{2} \mathrm{~N}_{-} \mathrm{CH}_{2}-\mathrm{CH}_{2}-\mathrm{NH}_{2}$$)
Match List I with List II
| List - I (Complex) | List - II (Hybridization) | ||
|---|---|---|---|
| (A) | $$Ni{(CO)_4}$$ | (I) | $$s{p^3}$$ |
| (B) | $${[Ni{(CN)_4}]^{2 - }}$$ | (II) | $$s{p^3}{d^2}$$ |
| (C) | $${[Co{(CN)_6}]^{3 - }}$$ | (III) | $${d^2}s{p^3}$$ |
| (D) | $${[Co{F_6}]^{3 - }}$$ | (IV) | $$ds{p^2}$$ |
Choose the correct answer from the options given below :
Low oxidation state of metals in their complexes are common when ligands :
$$\mathrm{Fe}^{3+}$$ cation gives a prussian blue precipitate on addition of potassium ferrocyanide solution due to the formation of :
The metal complex that is diamagnetic is (Atomic number: $$\mathrm{Fe}, 26 ; \mathrm{Cu}, 29)$$
The correct order of energy of absorption for the following metal complexes is :
A : [Ni(en)3]2+ , B : [Ni(NH3)6]2+ , C : [Ni(H2O)6]2+
Correct formula of the compound which gives a white precipitate with BaCl2 solution, but not with AgNO3 solution, is :
Given below are two statements.
$$\bullet$$ Statement I : In CuSO4 . 5H2O, Cu-O bonds are present.
$$\bullet$$ Statement II : In CuSO4 . 5H2O, ligands coordinating with Cu(II) ion are O-and S-based ligands.
In the light of the above statements, choose the correct answer from the options given below.
Given below are two statements :
Statement I : [Ni(CN)4]2$$-$$ is square planar and diamagnetic complex, with dsp2 hybridization for Ni but [Ni(CO)4] is tetrahedral, paramagnetic and with sp3-hybridication for Ni.
Statement II : [NiCl4]2$$-$$ and [Ni(CO)4] both have same d-electron configuration have same geometry and are paramagnetic.
In light the above statements, choose the correct answer from the options given below :
Arrange the following coordination compounds in the increasing order of magnetic moments. (Atomic numbers : Mn = 25; Fe = 26)
A. [FeF6]3$$-$$
B. [Fe(CN)6]3$$-$$
C. [MnCl6]3$$-$$ (high spin)
D. [Mn(CN)6]3$$-$$
Choose the correct answer from the options given below :
Which of the following will have maximum stabilization due to crystal field?
Which statement is not true with respect to nitrate ion test?
Transition metal complex with highest value of crystal field splitting ($$\Delta$$0) will be :
Match List - I with List - II :
| List - I | List -II | ||
|---|---|---|---|
| (A) | $${[PtC{l_4}]^{2 - }}$$ | (I) | $$s{p^3}d$$ |
| (B) | $$Br{F_5}$$ | (II) | $${d^2}s{p^3}$$ |
| (C) | $$PC{l_5}$$ | (III) | $$ds{p^2}$$ |
| (D) | $${[Co{(N{H_3})_6}]^{3 + }}$$ | (IV) | $$s{p^3}{d^2}$$ |
Choose the most appropriate answer from the options given below :
Complexes : $$\mathop {{{[Co{F_6}]}^{3 - }}}\limits_A ,\mathop {{{[Co{{({H_2}O)}_6}]}^{2 + }}}\limits_B ,\mathop {{{[Co{{(N{H_3})}_6}]}^{3 + }}}\limits_C and \mathop {{{[Co{{({en})}_3}]}^{3 + }}}\limits_D $$
Choose the correct option :
Statement I : $${[Mn{(CN)_6}]^{3 - }}$$, $${[Fe{(CN)_6}]^{3 - }}$$ and $${[Co{({C_2}{O_4})_3}]^{3 - }}$$ are d2sp3 hybridised.
Statement II : $${[MnCl)_6}{]^{3 - }}$$ and $${[Fe{F_6}]^{3 - }}$$ are paramagnetic and have 4 and 5 unpaired electrons, respectively.
In the light of the above statements, choose the correct answer from the options given below :
complexes [PtCl2(NH3)2], [Ni(CO)4], [Ru(H2O)3Cl3 and [CoCl2(NH3)4]+ respectively, are :
| List - I |
List - II |
||
|---|---|---|---|
| (a) | $$[Co{(N{H_3})_6}][Cr{(CN)_6}]$$ | (i) | Linkage isomerism |
| (b) | $$[Co{(N{H_3})_3}{(N{O_2})_3}]$$ | (ii) | Solvate isomerism |
| (c) | $$[Cr{({H_2}O)_6}C{l_3}$$ | (iii) | Co-ordination isomerism |
| (d) | $$cis - {[CrC{l_2}{(ox)_2}]^{3 - }}$$ | (iv) | Optical isomerism |
Choose the correct answer from the options given below :
(i) [FeF6]3$$-$$
(ii) [Co(NH3)6]3+
(iii) [NiCl4]2$$-$$
(iv) [Cu(NH3)4]2+
(Td = tetrahedral)
trans-[Co(en)2Cl2]+ (A) and
cis-[Co(en)2Cl2]+ (B).
The correct statement regarding them is :
(CFSE) of [CoF3(H2O)3] ($$\Delta $$0 < P) is :
gly = glycinato; bpy = 2, 2'-bipyridine
[Pt(en)(NO2)2] is :
and [Fe(H2O)6]Cl2 , respectively are :
[Given: atomic mass of Cr = 52 amu and Cl = 35 amu]
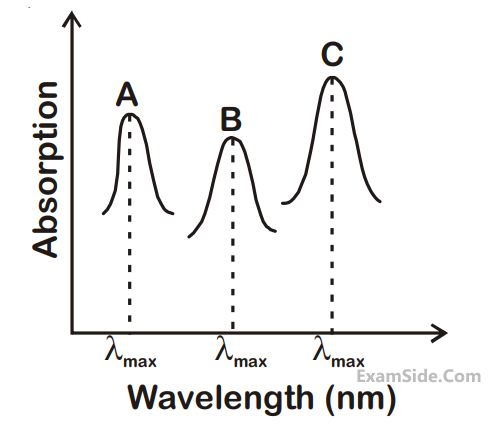
(i) [M(NCS)6](–6 + n)
(ii) [MF6](–6 + n)
(iii) [M(NH3)6]n+
(I) both the complexes can be high spin.
(II) Ni(II) complex can very rarely be low spin.
(III) with strong field ligands, Mn(II) complexes can be low spin.
(IV)aqueous solution of Mn(II) ions is yellow in colour.
The correct statements are :
(I) [Cr(H2O)6]Br2
(II) Na4[Fe(CN)6]
(III) Na3[Fe(C2O4)3] ($$\Delta $$0 $$>$$ P)
(IV) (Et4N)2[CoCl4]
[Note : Ignore the pairing energy]
(a) [Pt(NH3)3Cl]+
(b) [Pt(NH3)Cl5]–
(c) [Pt(NH3)2Cl(NO2)]
(d) [Pt(NH3)4ClBr]2+
(A) Ni(CO)4
(B) [Ni(H2O)6]Cl2
(C) Na2[Ni(CN)4]
(D) PdCl2(PPh3)2
Note : A and B are unidentate netural and unidentate monoanionic ligands, respectively.
(a) Octahedral CO(III) complexes with strong fields ligands have very high magnetic moments.
(b) When $$\Delta $$0 < P, the d-electron configuration of Co(III) in an octahedral complex is $$t_{eg}^4e_g^2$$
(c) Wavelength of light absorbed by [Co(en)3]3+ is lower than that of [CoF6]3-
(d) If the $$\Delta $$0 for an octahedral complex of CO(III) is 18,000 cm-1, the $$\Delta $$t for its tetrahedral complex with the same ligand be 16,000 cm-1
(en = ehane-1, 2-diamine, ox = oxalate)
[CoCl(NH3)5] 2+(I),
[Co(NH3)5H2O]3+ (II) and
[Co(NH3)6] 3+(III)
absorb light in the visible region. The correct order of the wavelength of light absorbed by them is :

(I) Valence bond theory cannot explain the color exhibited by transition metal complexes.
(II) Valence bond theory can predict quantitatively the magnetic properties of transtition metal complexes.
(III) Valence bond theory cannot distinguish ligands as weak and strong field ones.
(en = ethane-1,2-diamine)

(C2O$${_4^{2 - }}$$ = Oxalato)
| (Column I) Metals |
(Column II) Coordination compounds(s) enzyme(s) |
||
|---|---|---|---|
| (A) | Co | (i) | Wilkinson catalyst |
| (B) | Zn | (ii) | Chlorophyl |
| (C) | Rh | (iii) | Vitamin B12 |
| (D) | Mg | (iv) | Carbonic anhydrase |
[M(F)(Cl)(SCN)(NO2)] is
[Co(NH3)4Br2]+ + Br- $$\to$$ [Co(NH3)3Br3] + NH3
(I) Two isomers are produced if the reactant complex ion is a cis-isomer
(II) Two isomers are produced if the reactant complex ion is a trans-isomer
(III) Only one isomer is produced if the reactant complex ion is a trans-isomer
(IV) Only one isomer is produced if the reactant complex ion is a cis – isomer
The correct statements are
(Atomic number : Mn = 25, Co = 27, Ni = 28, Zn = 30)
[At. No.: Cr = 24, Mn = 25, Fe = 26, Co = 27]
(en = ethylenediamine)
(en = ethylenediamine)
(At. No. Cr = 24, Mn = 25, Fe = 26, Co = 27)
(Atomic numbers: Mn = 25; Fe = 26, Co =27)
Numerical
The number of paramagnetic complexes among $\left[\mathrm{FeF}_6\right]^{3-},\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-},\left[\mathrm{Mn}(\mathrm{CN})_6\right]^{3-}$, $\left[\mathrm{Co}\left(\mathrm{C}_2 \mathrm{O}_4\right)_3\right]^{3-},\left[\mathrm{MnCl}_6\right]^{3-}$, and $\left[\mathrm{CoF}_6\right]^{3-}$, which involved $\mathrm{d}^2 \mathrm{sp}^3$ hybridization is _________.
A metal complex with a formula $\mathrm{MCl}_4 \cdot 3 \mathrm{NH}_3$ is involved in $\mathrm{sp}^3 \mathrm{~d}^2$ hybridisation. It upon reaction with excess of $\mathrm{AgNO}_3$ solution gives ' $x$ ' moles of AgCl . Consider ' $x$ ' is equal to the number of lone pairs of electron present in central atom of $\mathrm{BrF}_5$. Then the number of geometrical isomers exhibited by the complex is _________.
The number of optical isomers exhibited by the iron complex $(\mathrm{A})$ obtained from the following reaction is___________.
$$ \mathrm{FeCl}_3+\mathrm{KOH}+\mathrm{H}_2 \mathrm{C}_2 \mathrm{O}_4 \rightarrow \mathrm{~A} $$
The spin-only magnetic moment value of $\mathrm{M}^{\mathrm{n}+}$ ion formed among $\mathrm{Ni}, \mathrm{Zn}, \mathrm{Mn}$ and Cu that has the least enthalpy of atomisation is_________ . (in nearest integer) Here n is equal to the number of diamagnetic complexes among $\mathrm{K}_2\left[\mathrm{NiCl}_4\right],\left[\mathrm{Zn}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right] \mathrm{Cl}_2$, $\mathrm{K}_3\left[\mathrm{Mn}(\mathrm{CN})_6\right]$ and $\left[\mathrm{Cu}\left(\mathrm{PPh}_3\right)_3 \mathrm{I}\right]$
A transition metal (M) among $\mathrm{Mn}, \mathrm{Cr}, \mathrm{Co}$ and Fe has the highest standard electrode potential $\left(\mathrm{M}^{3+} / \mathrm{M}^{2+}\right)$. It forms a metal complex of the type $\left[\mathrm{M}(\mathrm{CN})_6\right]^{4-}$. The number of electrons present in the $\mathrm{e}_{\mathrm{g}}$ orbital of the complex is ___________.
Consider the following low-spin complexes
$$ \mathrm{K}_3\left[\mathrm{Co}\left(\mathrm{NO}_2\right)_6\right], \mathrm{K}_4\left[\mathrm{Fe}(\mathrm{CN})_6\right], \mathrm{K}_3\left[\mathrm{Fe}(\mathrm{CN})_6\right], \mathrm{Cu}_2\left[\mathrm{Fe}(\mathrm{CN})_6\right] \text { and } \mathrm{Zn}_2\left[\mathrm{Fe}(\mathrm{CN})_6\right] $$
The sum of the spin-only magnetic moment values of complexes having yellow colour is ________ B.M. (answer in nearest integer)
$\mathrm{O}_2, \mathrm{O}_2^{+}, \mathrm{O}_2^{-}, \mathrm{NO}, \mathrm{NO}_2, \mathrm{CO}, \mathrm{K}_2\left[\mathrm{NiCl}_4\right],\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_6\right] \mathrm{Cl}_3, \mathrm{~K}_2\left[\mathrm{Ni}(\mathrm{CN})_4\right]$
The complex of $\mathrm{Ni}^{2+}$ ion and dimethyl glyoxime contains __________ number of Hydrogen (H) atoms.
Consider the following test for a group-IV cation.
$$\mathrm{M}^{2+}+\mathrm{H}_2 \mathrm{S} \rightarrow \mathrm{A} \text { (Black precipitate)+ byproduct }$$
$$\mathrm{A}+\text { aqua regia } \rightarrow \mathrm{B}+\mathrm{NOCl}+\mathrm{S}+\mathrm{H}_2 \mathrm{O}$$
$$\mathrm{B}+\mathrm{KNO}_2+\mathrm{CH}_3 \mathrm{COOH} \rightarrow \mathrm{C}+\text { byproduct }$$
The spin-only magnetic moment value of the metal complex $$\mathrm{C}$$ is _________ $$\mathrm{BM}$$ (Nearest integer)
Number of ambidentate ligands among the following is _________.
$$\mathrm{NO}_2^{-}, \mathrm{SCN}^{-}, \mathrm{C}_2 \mathrm{O}_4^{2-}, \mathrm{NH}_3, \mathrm{CN}^{-}, \mathrm{SO}_4^{2-}, \mathrm{H}_2 \mathrm{O} \text {. }$$
Total number of unpaired electrons in the complex ions $$[\mathrm{Co}(\mathrm{NH}_3)_6]^{3+}$$ and $$[\mathrm{NiCl}_4]^{2-}$$ is ________.
The 'spin only' magnetic moment value of $$\mathrm{MO}_4{ }^{2-}$$ is ________ BM. (Where M is a metal having least metallic radii. among $$\mathrm{Sc}, \mathrm{Ti}, \mathrm{V}, \mathrm{Cr}, \mathrm{Mn}$$ and $$\mathrm{Zn}$$ ).
(Given atomic number: $$\mathrm{Sc}=21, \mathrm{Ti}=22, \mathrm{~V}=23, \mathrm{Cr}=24, \mathrm{Mn}=25$$ and $$\mathrm{Zn}=30$$)
The difference in the 'spin-only' magnetic moment values of $$\mathrm{KMnO}_4$$ and the manganese product formed during titration of $$\mathrm{KMnO}_4$$ against oxalic acid in acidic medium is ________ $$\mathrm{BM}$$. (nearest integer)
The spin-only magnetic moment value of the ion among $$\mathrm{Ti}^{2+}, \mathrm{V}^{2+}, \mathrm{Co}^{3+}$$ and $$\mathrm{Cr}^{2+}$$, that acts as strong oxidising agent in aqueous solution is _________ BM (Near integer).
(Given atomic numbers : $$\mathrm{Ti}: 22, \mathrm{~V}: 23, \mathrm{Cr}: 24, \mathrm{Co}: 27$$)
The 'Spin only' Magnetic moment for $$\left[\mathrm{Ni}\left(\mathrm{NH}_3\right)_6\right]^{2+}$$ is _________ $$\times 10^{-1} \mathrm{~BM}$$. (given $$=$$ Atomic number of $$\mathrm{Ni}: 28$$)
Number of complexes which show optical isomerism among the following is ________.
$$\text { cis- }\left[\mathrm{Cr}(\mathrm{ox})_2 \mathrm{Cl}_2\right]^{3-},\left[\mathrm{Co}(\text {en})_3\right]^{3+}, \text { cis- }\left[\mathrm{Pt}(\text {en})_2 \mathrm{Cl}_2\right]^{2+}, \text { cis- }\left[\mathrm{Co}(\text {en})_2 \mathrm{Cl}_2\right]^{+}, \text {trans- }\left[\mathrm{Pt}(\text {en})_2 \mathrm{Cl}_2\right]^{2+}, \text { trans- }\left[\mathrm{Cr}(\mathrm{ox})_2 \mathrm{Cl}_2\right]^{3-}$$
The Spin only magnetic moment value of square planar complex $$\left[\mathrm{Pt}\left(\mathrm{NH}_3\right)_2 \mathrm{Cl}\left(\mathrm{NH}_2 \mathrm{CH}_3\right)\right] \mathrm{Cl}$$ is _________ B.M. (Nearest integer)
(Given atomic number for $$\mathrm{Pt}=78$$)
The ratio of spin-only magnetic moment values $$\mu_{\text {eff }}\left[\mathrm{Cr}(\mathrm{CN})_{6}\right]^{3-} / \mu_{\text {eff }}\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{3+}$$ is _________.
For a metal ion, the calculated magnetic moment is $$4.90 ~\mathrm{BM}$$. This metal ion has ___________ number of unpaired electrons.
In potassium ferrocyanide, there are ________ pairs of electrons in the $$t_{2g}$$ set of orbitals.
The observed magnetic moment of the complex $$\left.\left[\operatorname{Mn}(\underline{N} C S)_{6}\right)\right]^{x^{-}}$$ is $$6.06 ~\mathrm{BM}$$. The numerical value of $$x$$ is __________.
Number of ambidentate ligands in a representative metal complex $$\left[\mathrm{M}(\mathrm{en})(\mathrm{SCN})_{4}\right]$$ is ___________.
[en = ethylenediamine]
The spin only magnetic moment of $$\left[\mathrm{Mn}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}$$ complexes is _________ B.M. (Nearest integer)
(Given : Atomic no. of Mn is 25)
Assume Planck's constant (h) $=6.4 \times 10^{-34} \mathrm{Js}$, Speed of light $(\mathrm{c})=3.0 \times 10^{8} \mathrm{~m} / \mathrm{s}$ and Avogadro's
Constant $\left(\mathrm{N}_{\mathrm{A}}\right)=6 \times 10^{23} / \mathrm{mol}$
The sum of bridging carbonyls in $$\mathrm{W(CO)_6}$$ and $$\mathrm{Mn_2(CO)_{10}}$$ is ____________.
Total number of moles of AgCl precipitated on addition of excess of AgNO$$_3$$ to one mole each of the following complexes $$\mathrm{[Co(NH_3)_4Cl_2]Cl,[Ni(H_2O)_6]Cl_2,[Pt(NH_3)_2Cl_2]}$$ and $$\mathrm{[Pd(NH_3)_4]Cl_2}$$ is ___________.
The number of paramagnetic species from the following is _____________.
$$\mathrm{{[Ni{(CN)_4}]^{2 - }},[Ni{(CO)_4}],{[NiC{l_4}]^{2 - }}}$$
$$\mathrm{{[Fe{(CN)_6}]^{4 - }},{[Cu{(N{H_3})_4}]^{2 + }}}$$
$$\mathrm{{[Fe{(CN)_6}]^{3 - }}\,and\,{[Fe{({H_2}O)_6}]^{2 + }}}$$
The d-electronic configuration of $$\mathrm{[CoCl_4]^{2-}}$$ in tetrahedral crystal field in $${e^mt_2^n}$$. Sum of "m" and "number of unpaired electrons" is ___________
Sum of oxidation state (magnitude) and coordination number of cobalt in $$\mathrm{Na}\left[\mathrm{Co}(\mathrm{bpy}) \mathrm{Cl}_{4}\right]$$ is _________.

$$\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}$$ should be an inner orbital complex. Ignoring the pairing energy, the value of crystal field stabilization energy for this complex is $$(-)$$ ____________ $$\Delta_{0}$$. (Nearest integer)
Total number of relatively more stable isomer(s) possible for octahedral complex $$\left[\mathrm{Cu}(\mathrm{en})_{2}(\mathrm{SCN})_{2}\right]$$ will be _________.
The conductivity of a solution of complex with formula $$\mathrm{CoCl}_{3}\left(\mathrm{NH}_{3}\right)_{4}$$ corresponds to 1 : 1 electrolyte, then the primary valency of central metal ion is __________.
The difference between spin only magnetic moment values of $$\left[\mathrm{Co}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]\mathrm{Cl}_{2}$$ and $$\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{3}$$ is ___________.
Consider the following metal complexes :
$$\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}$$
$$\left[\mathrm{CoCl}\left(\mathrm{NH}_{3}\right)_{5}\right]^{2+}$$
$$\left[\mathrm{Co}(\mathrm{CN})_{6}\right]^{3-}$$
$$\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{5}\left(\mathrm{H}_{2} \mathrm{O}\right)\right]^{3+}$$
The spin-only magnetic moment value of the complex that absorbes light with shortest wavelength is _____________ B. M. (Nearest integer)
In the following brown complex, the oxidation state of iron is +_____________.
$${[Fe{({H_2}O)_6}]^{2 + }} + NO \to \mathop {{{[Fe{{({H_2}O)}_5}(NO)]}^{2 + }}}\limits_{\text{Brown complex}} + {H_2}O$$
Spin only magnetic moment ($$\mu$$s) of $${K_3}[Fe{(CN)_6}]$$ is ____________ B.M.
(Nearest integer)
$${[Fe{(CN)_6}]^{4 - }}$$
$${[Fe{(CN)_6}]^{3 - }}$$
$${[Ti{(CN)_6}]^{3 - }}$$
$${[Ni{(CN)_4}]^{2 - }}$$
$${[Co{(CN)_6}]^{3 - }}$$
Among the given complexes, number of paramagnetic complexes is ____________.
(a) CoCl3.4NH3, (b) CoCl3.5NH3, (c) CoCl3.6NH3 and (d) CoCl(NO3)2.5NH3.
Number of complex(es) which will exist in cis-trans form is/are _______________.
Number of complexes which will exhibit synergic bonding amongst, $$[Cr{(CO)_6}]$$, $$[Mn{(CO)_5}]$$ and $$[M{n_2}{(CO)_{10}}]$$ is ___________.
Acidified potassium permanganate solution oxidises oxalic acid. The spin-only magnetic moment of the manganese product formed from the above reaction is ____________ B.M. (Nearest integer)
Reaction of [Co(H2O)6]2+ with excess ammonia and in the presence of oxygen results into a diamagnetic product. Number of electrons present in t2g-orbitals of the product is ___________.
The spin-only magnetic moment value of an octahedral complex among CoCl3.4NH3, NiCl2.6H2O and PtCl4.2HCl, which upon reaction with excess of AgNO3 gives 2 moles of AgCl is ___________ B.M. (Nearest integer)
Amongst FeCl3.3H2O, K3[Fe(CN)6] and [Co(NH3)6]Cl3, the spin-only magnetic moment value of the inner-orbital complex that absorbs light at shortest wavelength is ____________ B.M. [nearest integer]
If [Cu(H2O)4]2+ absorbs a light of wavelength 600 nm for d-d transition, then the value of octahedral crystal field splitting energy for [Cu(H2O)6]2+ will be ____________ $$\times$$ 10$$-$$21 J. [Nearest Integer]
(Given : h = 6.63 $$\times$$ 10$$-$$34 Js and c = 3.08 $$\times$$ 108 ms$$-$$1)
In the cobalt-carbonyl complex : [Co2(CO)8], number of Co-Co bonds is "X" and terminal CO ligands is "Y". X + Y = ___________.
(Round off to the nearest integer)
[At. no. of Co = 27]
(in BM) of [Ru(H2O)6]2+ would be _________.
in ethylenediaminetetraacetate (EDTA4–) is _____.
Na4[Fe(CN)5(NOS)]
(A)
Na4[FeO4]
(B)
[Fe2(CO)9]
(C)