Alcohols, Phenols and Ethers · Chemistry · JEE Main
MCQ (Single Correct Answer)
Given below are two statements :
Statement I : Dimethyl ether is completely soluble in water. However, diethyl ether is soluble in water to a very small extent.
Statement II : Sodium metal can be used to dry diethyl ether and not ethyl alcohol.
In the light of given statements. choose the correct answer from the options given below
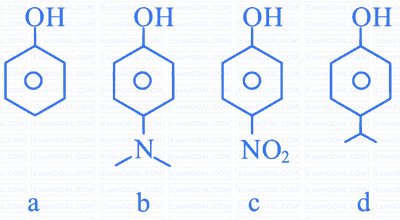
Benzene is treated with oleum to produce compound $(\mathrm{X})$ which when further heated with molten sodium hydroxide followed by acidification produces compound $(\mathrm{Y})$. The compound Y is treated with zinc metal to produce compound $(Z)$. Identify the structure of compound $(Z)$ from the following option.
Which one of the following, with HBr will give a phenol?
Given below are two statements :
Statement I : 
Statement II : In  , intramolecular substitution takes place first by involving lone pair of electrons on nitrogen.
, intramolecular substitution takes place first by involving lone pair of electrons on nitrogen.
In the light of the above statements, choose the most appropriate answer from the options given below
Given below are two statements :
Statement (I) : The boiling points of alcohols and phenols increase with increase in the number of C -atoms.
Statement (II) : The boiling points of alcohols and phenols are higher in comparison to other class of compounds such as ethers, haloalkanes.
In the light of the above statements, choose the correct answer from the options given below :
What amount of bromine will be required to convert 2 g of phenol into 2,4,6-tribromophenol?
(Given molar mass in $\mathrm{g} \mathrm{mol}^{-1}$ of $\mathrm{C}, \mathrm{H}, \mathrm{O}, \mathrm{Br}$ are $12,1,16,80$ respectively )
Match List I with List II

Choose the correct answer from the options given below :
Which one the following compounds will readily react with dilute $$\mathrm{NaOH}$$ ?
Identify the major products A and B respectively in the following set of reactions.

The major products formed :

A and B respectively are :
In Reimer - Tiemann reaction, phenol is converted into salicylaldehyde through an intermediate. The structure of intermediate is __________.

Consider the above reaction sequence and identify the major product P.
Which one of the following reactions is NOT possible?
Given below are two statement :
Statements I : Bromination of phenol in solvent with low polarity such as $$\mathrm{CHCl}_3$$ or $$\mathrm{CS}_2$$ requires Lewis acid catalyst.
Statements II : The Lewis acid catalyst polarises the bromine to generate $$\mathrm{Br}^{+}$$.
In the light of the above statements, choose the correct answer from the options given below :
For the Compounds :
(A) $$\mathrm{H}_3 \mathrm{C}-\mathrm{CH}_2-\mathrm{O}-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{CH}_3$$
(B) $$\mathrm{H}_3 \mathrm{C}-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{CH}_3$$
(C) 
(D) 
The increasing order of boiling point is :
Choose the correct answer from the options given below :
Common name of Benzene - 1,2 - diol is -
| List I (Reactants) | List II (Product) |
|---|---|
| (A) Phenol, Zn/Δ | (I) Salicylaldehyde |
| (B) Phenol, CHCl3, NaOH, HCl | (II) Salicylic acid |
| (C) Phenol, CO2, NaOH, HCl | (III) Benzene |
| (D) Phenol, Conc. HNO3 | (IV) Picric acid |
Choose the correct answer from the options given below :
Given below are two statements: One is labelled as Assertion A and the other is labelled as Reason R:
Assertion A: Alcohols react both as nucleophiles and electrophiles.
Reason R: Alcohols react with active metals such as sodium, potassium and aluminum to yield corresponding alkoxides and liberate hydrogen.
In the light of the above statements, choose the correct answer from the options given below:
Given below are two statements: One is labelled as Assertion A and the other is labelled as Reason R:
Assertion A: $$\mathrm{pK}_{\mathrm{a}}$$ value of phenol is 10.0 while that of ethanol is 15.9 .
Reason R: Ethanol is stronger acid than phenol.
In the light of the above statements, choose the correct answer from the options given below:
Phenol treated with chloroform in presence of sodium hydroxide, which further hydrolyzed in presence of an acid results
Match List I with List II
| List - I (Compound) |
List - II ($$\mathrm{pK_a}$$ value) |
||
|---|---|---|---|
| (A) | Ethanol | (I) | 10.0 |
| (B) | Phenol | (II) | 15.9 |
| (C) | m-Nitrophenol | (III) | 7.1 |
| (D) | p-Nitrophenol | (IV) | 8.3 |
Choose the correct answer from the options given below:
The major product(P) in the following reaction is

Major product formed in the following reaction is a mixture of :

Phenolic group can be identified by a positive:
Match List - I with List - II.
| List - I (Reaction) |
List - II (Reagent(s)) |
||
|---|---|---|---|
| (A) |  |
(I) | $$\mathrm{Na_2Cr_2O_7,H_2SO_4}$$ |
| (B) |  |
(II) | (i) $$\mathrm(NaOH)$$, (ii) $$\mathrm{CH_3Cl}$$ |
| (C) |  |
(III) | (i) $$\mathrm{NaOH,CHCl_3}$$, (ii) $$\mathrm{NaOH}$$, (iii) $$\mathrm{HCl}$$ |
| (D) |  |
(IV) | (i) $$\mathrm{NaOH}$$, (ii) $$\mathrm{CO_2}$$, (iii) $$\mathrm{HCl}$$ |
Choose the correct answer from the options given below :
Given below are two statements :
Statement (I) : p-nitrophenol is more acidic than m-nitrophenol and o-nitrophenol.
Statement (II) : Ethanol will give immediate turbidity with Lucas reagent.
In the light of the above statements, choose the correct answer from the options given below :
The ascending order of acidity of $$-\mathrm{OH}$$ group in the following compounds is :


Choose the correct answer from the options given below:

'A' formed in the above reaction is
The major product for the following reaction is:

Given below are two statements, one is labelled as Assertion A and the other is labelled as Reason R.
Assertion A : Order of acidic nature of the following compounds is A > B > C.

Reason R : Fluoro is a stronger electron withdrawing group than Chloro group.
In the light of the above statements, choose the correct answer from the options given below:
2-Methyl propyl bromide reacts with $$\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{O}^{-}$$ and gives 'A' whereas on reaction with $$\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}$$ it gives 'B'. The mechanism followed in these reactions and the products 'A' and 'B' respectively are :
Correct statements for the given reaction are :

A. Compound '$$\mathrm{B}$$' is aromatic
B. The completion of above reaction is very slow
C. 'A' shows tautomerism
D. The bond lengths of C-C in compound $B$ are found to be same
Choose the correct answer from the options given below:
Compound 'B' is


Product [X] formed in the above reaction is :
Arrange the following compounds in increasing order of rate of aromatic electrophilic substitution reaction

The correct order for acidity of the following hydroxyl compound is :
A. $$\mathrm{CH}_{3} \mathrm{OH}$$
B. $$\left(\mathrm{CH}_{3}\right)_{3} \mathrm{COH}$$
C. 
D. 
E. 
Choose the correct answer from the options given below:
Incorrect method of preparation for alcohols from the following is:
Suitable reaction condition for preparation of Methyl phenyl ether is
Given below are two statements: One is labelled as Assertion A and the other is labelled as Reason R.
Assertion A: Butan -1- ol has higher boiling point than ethoxyethane.
Reason R: Extensive hydrogen bonding leads to stronger association of molecules.
In the light of the above statements, choose the correct answer from the options given below:
The water gas on reacting with cobalt as a catalyst forms
The strongest acid from the following is
The graph which represents the following reaction is :

Identify the incorrect option from the following
Decreasing order of dehydration of the following alcohols is

An organic compound 'A' with emperical formula $$\mathrm{C}_{6} \mathrm{H}_{6} \mathrm{O}$$ gives sooty flame on burning. Its reaction with bromine solution in low polarity solvent results in high yield of B. B is
A solution of $$\mathrm{Cr O_5}$$ in amyl alcohol has a __________ colour.
Find out the major product for the following reaction.

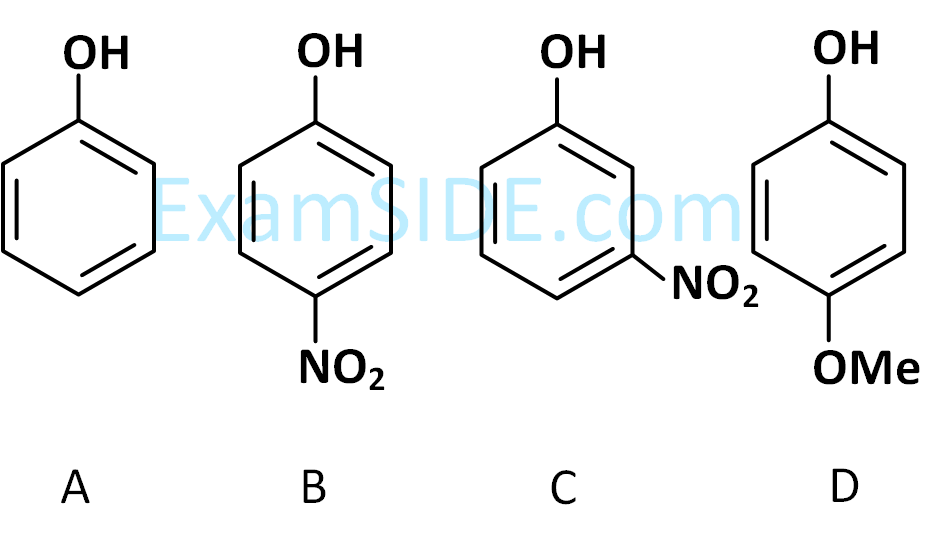
The increasing order of $$\mathrm{pK_a}$$ for the following phenols is
(A) 2, 4 - Dinitrophenol
(B) 4 - Nitrophenol
(C) 2, 4, 5 - Trimethylphenol
(D) Phenol
(E) 3-Chlorophenol
Choose the correct answer from the option given below :
Find out the major product from the following reaction.

Given below are two statements, one is labelled as Assertion A and the other is labelled as Reason R
Assertion A : Butylated hydroxy anisole when added to butter increases its shelf life.
Reason R : Butylated hydroxy anisole is more reactive towards oxygen than food.
In the light of the above statements, choose the most appropriate answer from the options given below
In the cumene to phenol preparation in presence of air, the intermediate is
Given below are two statements : one is labelled as Assertion A and the other is labelled as Reason R :
Assertion A : Acetal / Ketal is stable in basic medium.
Reason R : The high leaving tendency of alkoxide ion gives the stability to acetal/ketal in basic medium.
In the light of the above statements, choose the correct answer from the options given below :
'A' and 'B' formed in the following set of reactions are :

When ethanol is heated with conc. $$\mathrm{H}_{2} \mathrm{SO}_{4}$$, a gas is produced. The compound formed, when this gas is treated with cold dilute aqueous solution of Baeyer's reagent, is
A compound 'X' is acidic and it is soluble in NaOH solution, but insoluble in NaHCO3 solution. Compound 'X' also gives violet colour with neutral FeCl3 solution. The compound 'X' is :
Compound I is heated with Conc. HI to give a hydroxy compound A which is further heated with Zn dust to give compound B. Identify A and B.

Choose the correct option for the following reactions.

Identify the major products A and B for the below given reaction sequence.


In the above reaction product B is :
Hydrolysis of which compound will give carbolic acid?
The difference in the reaction of phenol with bromine in chloroform and bromine in water medium is due to :
Which reactant will give the following alcohol on reaction with one mole of phenyl magnesium bromide $$(\mathrm{PhMgBr})$$ followed by acidic hydrolysis?

The major product of the following reaction is

Most stable product of the following reaction is:

Given below are two statements :
Statement I : On heating with $$\mathrm{KHSO}_{4}$$, glycerol is dehydrated and acrolein is formed.
Statement II : Acrolein has fruity odour and can be used to test glycerol's presence.
Choose the correct option.

Consider the above reaction sequence. Identify the component A and component B :
Given below are two statements :
Statement I : Phenols are weakly acidic.
Statement II : Therefore they are freely soluble in NaOH solution and are weaker acids than alcohols and water.
Choose the most appropriate option :
The major product (P) of the given reaction is
(where, Me is $$-$$CH3)

Match List-I with List-II.
| List-I | List-II | ||
|---|---|---|---|
| (A) |  |
I. | $$B{r_2}$$ in $$C{S_2}$$ |
| (B) |  |
II. | $$N{a_2}C{r_2}{O_7}/{H_2}S{O_4}$$ |
| (C) |  |
III. | $$Zn$$ |
| (D) |  |
IV. | $$CHC{l_3}/NaOH$$ |
Choose the correct answer from the options given below :

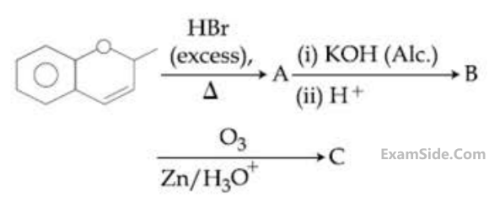
Consider the above reaction sequence and identify the product B.
In the given reaction

'A' can be
The major product formed in the following reaction, is

The intermediate X, in the reaction :

In the following reaction :

The compounds A and B respectively are :
Hex-4-ene-2-ol on treatment with PCC gives 'A'. 'A' on reaction with sodium hypoiodite gives 'B', which on further heating with soda lime gives 'C'. The compound 'C' is :

Assertion (A) : Treatment of bromine water with propene yields 1-bromopropan-2-ol.
Reason (R) : Attack of water on bromonium ion follows Markovnikov rule and results in 1-bromopropan-2-ol.
In the light of the above statements, choose the most appropriate answer from the options given below :
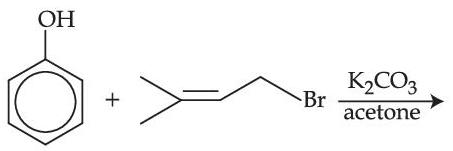

Assertion (A) : Synthesis of ethyl phenyl ether may be achieved by Williamson synthesis.
Reason (R) : Reaction of bromobenzene with sodium ethoxide yields ethyl phenyl ether.
In the light of the above statements, choose the most appropriate answer from the options given below :


Consider the above reaction, the major product "P" formed is :

Consider the above reaction, and choose the correct statement :
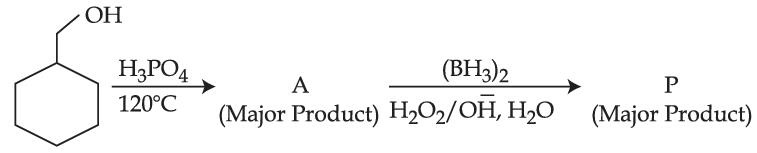
Consider the above reaction and identify the Product P :
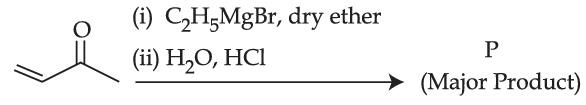
Consider the above reaction, the major product 'P' is :
3-Bromo-2, 2-dimethyl butane $$\buildrel {{C_2}{H_5}OH} \over \longrightarrow \mathop {'A'}\limits_{(Major\,\Pr oduct)} $$
Product A is :
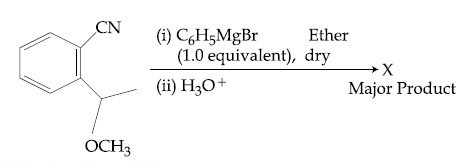
The structure of X is :


Statement I : o-Nitrophenol is steam volatile due to intramolecular hydrogen bonding.
Statement II : o-Nitrophenol has high melting due to hydrogen bonding.
In the light of the above statements, choose the most appropriate answer from the options given below :

B reacts with Hydroxyl amine but does not give Tollen's test. Identify A and B.


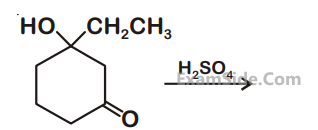



The product 'P' gives positive ceric ammonium nitrate test. This is because of the presence of which of these –OH group(s)?
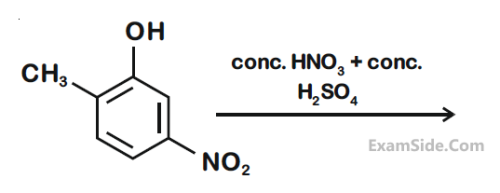
methanol, phenol, p-ethoxyphenol


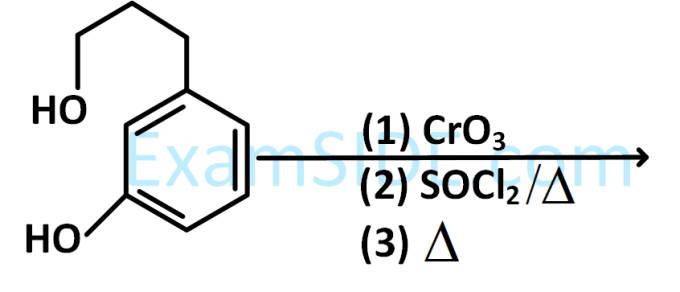

$${\rm{C}}{{\rm{H}}_3}{\rm{CH = CHC}}{{\rm{O}}_2}{\rm{CH_3 }}\buildrel {LiAl{H_4}} \over \longrightarrow $$



can not be prepared by -
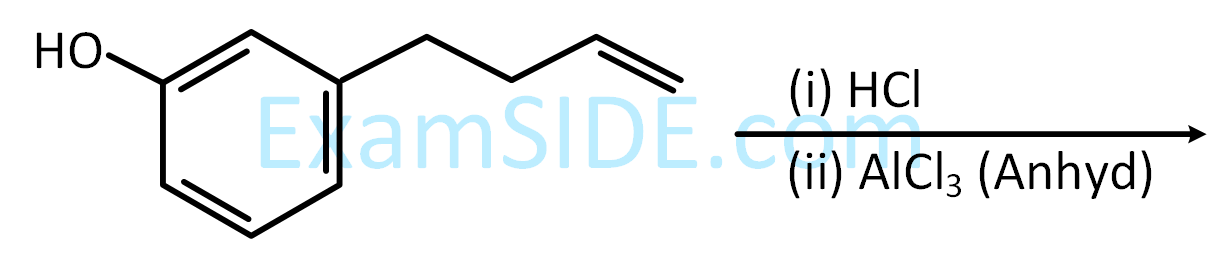



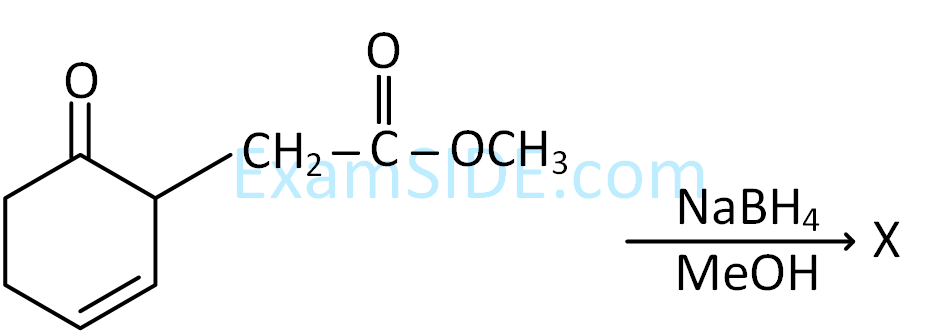








CH3CH2OH $$\buildrel {P + {I_2}} \over \longrightarrow $$ A $$\mathrel{\mathop{\kern0pt\longrightarrow} \limits_{ether}^{Mg}} $$ B $$\buildrel {HCHO} \over \longrightarrow $$ C $$\buildrel {{H_2}O} \over \longrightarrow $$ D
the compound ‘D’ is
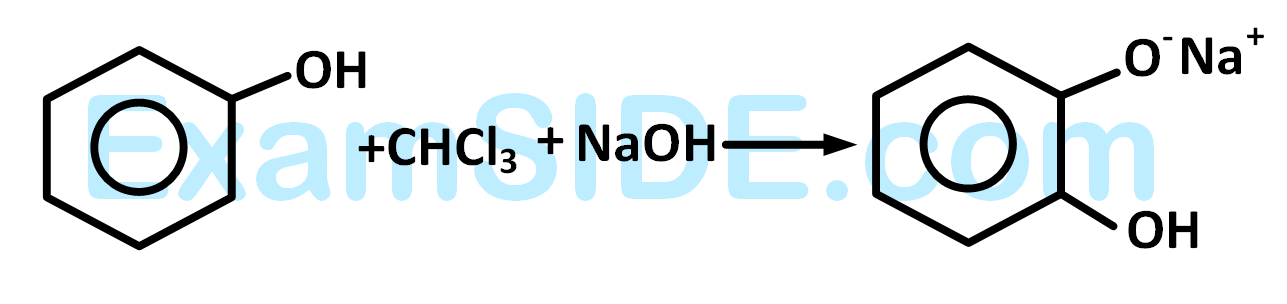
The electrophile involved in the above reaction is
Numerical

0.1 mole of compound ‘S’ will weigh ___________ g.
(Given molar mass in g mol−1 C : 12, H : 1, O : 16)
Consider the given chemical reaction sequence :

Total sum of oxygen atoms in Product A and Product B are ________.

The number of hyperconjugation structures involved to stabilize carbocation formed in the above reaction is _________.

The ratio x/y on completion of the above reaction is __________.
Among the following, the number of compounds which will give positive iodoform reaction is _________
(a) 1-Phenylbutan-2-one
(b) 2-Methylbutan-2-ol
(c) 3-Methylbutan-2-ol
(d) 1-Phenylethanol
(e) 3,3-dimethylbutan-2-one
(f) 1-Phenylpropan-2-ol
The denticity of the ligand present in the Fehling's reagent is ___________.
In the following reaction

The $$\%$$ yield for reaction I is $$60 \%$$ and that of reaction II is $$50 \%$$. The overall yield of the complete reaction is __________ $$\%$$. [nearest integer]
The number of chiral alcohol(s) with molecular formula C4H10O is ________.
In the given reaction,

the number of sp2 hybridised carbon(s) in compound 'X' is ________.
Compound 'P' on nitration with dil. HNO3 yields two isomers (A) and (B). These isomers can be separated by steam distillation. Isomers (A) and (B) show the intramolecular and intermolecular hydrogen bonding respectively. Compound (P) on reaction with conc. HNO3 yields a yellow compound 'C', a strong acid. The number of oxygen atoms is present in compound 'C' _____________.
(Atomic mass : C = 12; H = 1; O = 16)