Haloalkanes and Haloarenes · Chemistry · JEE Main
MCQ (Single Correct Answer)

'P' is
Choose the correct set of reagents for the following conversion.

Which one of the following reactions will not lead to the desired ether formation in major proportion?
(iso- $\mathrm{Bu} \Rightarrow$ isobutyl, sec $-\mathrm{Bu} \Rightarrow$ sec-butyl, $\mathrm{nPr} \Rightarrow \mathrm{n}$-propyl, $$ { }^{\mathrm{t}} \mathrm{Bu} \Rightarrow \text { tert-butyl, } \mathrm{Et} \Rightarrow \text { ethyl) } $$
Consider the following molecule(X).

The major product formed when the given molecule $(X)$ is treated with $\mathrm{HBr}(1 \mathrm{eq})$ is :
Given below are two statements :
Statement (I) : Alcohols are formed when alkyl chlorides are treated with aqueous potassium hydroxide by elimination reaction.
Statement (II) : In alcoholic potassium hydroxide, alkyl chlorides form alkenes by abstracting the hydrogen from the $\beta$-carbon.
In the light of the above statements, choose the most appropriate answer from the options given below :
$$ \text {In the following series of reactions identify the major products } \mathrm{A} \& \mathrm{~B} \text { respectively } $$

Which among the following halides will generate the most stable carbocation in the nucleophilic substitution reaction?
The major product of the following reaction is:

The products A and B in the following reactions, respectively are
$\mathrm{A} \stackrel{\mathrm{Ag}-\mathrm{NO}_2}{\longleftrightarrow} \mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{Br} \xrightarrow{\mathrm{AgCN}} \mathrm{B}$
The structure of the major product formed in the following reaction is :

Given below are two statements:
Statement I: The conversion proceeds well in the less polar medium.
$$\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{Cl} \xrightarrow{\mathrm{HO}^{-}} \mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{OH}+\mathrm{Cl}^{(-)}$$
Statement II: The conversion proceeds well in the more polar medium.

In the light of the above statements, choose the correct answer from the options given below
Following are the four molecules "P", "Q", "R" and "S".
Which one among the four molecules will react with $\mathrm{H}-\mathrm{Br}_{(\mathrm{aq})}$ at the fastest rate?

Identify the products [A] and [B], respectively in the following reaction:

The ascending order of relative rate of solvolysis of following compounds is :

Propane molecule on chlorination under photochemical condition gives two di-chloro products, " $x$ " and " $y$ ". Amongst " $x$ " and " $y$ ", " $x$ " is an optically active molecule. How many tri-chloro products (consider only structural isomers) will be obtained from " x " when it is further treated with chlorine under the photochemical condition?

The maximum number of RBr producing 2-methylbutane by above sequence of reactions is __________ . (Consider the structural isomers only)
Given below are two statements :
Statement I : $\quad \mathrm{CH}_3-\mathrm{O}-\mathrm{CH}_2-\mathrm{Cl}$ will undergo $\mathrm{S}_{\mathrm{N}} 1$ reaction though it is a primary halide.
Statement II :  will not undergo $\mathrm{S}_{\mathrm{N}} 2$ reaction very easily though it is a primary halide.
will not undergo $\mathrm{S}_{\mathrm{N}} 2$ reaction very easily though it is a primary halide.
In the light of the above statements, choose the most appropriate answer from the options given below :
Given below are two statements : one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A) : $$\mathrm{S}_{\mathrm{N}} 2$$ reaction of $$\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_2 \mathrm{Br}$$ occurs more readily than the $$\mathrm{S}_{\mathrm{N}} 2$$ reaction of $$\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{Br}$$.
Reason (R) : The partially bonded unhybridized p-orbital that develops in the trigonal bipyramidal transition state is stabilized by conjugation with the phenyl ring.
In the light of the above statements, choose the most appropriate answer from the options given below :
In the following sequence of reaction, the major products B and C respectively are :

Which among the following compounds will undergo fastest SN2 reaction.
Identify the major product in the following reaction.


Product P is
$$\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{Br}+\mathrm{NaOH} \xrightarrow{\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}} \text { Product 'A' }$$

Consider the above reactions, identify product B and product C.
Find out the major product formed from the following reaction. $$[\mathrm{Me}:-\mathrm{CH}_3]$$


Identify B and C and how are A and C related?
Identify the correct set of reagents or reaction conditions '$$X$$' and '$$Y$$' in the following set of transformation.

Assertion (A) : Haloalkanes react with KCN to form alkyl cyanides as a main product while with $\mathrm{AgCN}$ form isocyanide as the main product.
Reason (R) : $\mathrm{KCN}$ and $\mathrm{AgCN}$ both are highly ionic compounds.
In the light of the above statements, choose the most appropriate answer from the options given below :
Identify A and B in the following reaction sequence.

The product (C) in the below mentioned reaction is :
$$\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{Br} \xrightarrow[\Delta]{\mathrm{KOH}_{(\text {alc) }}} \mathrm{A} \xrightarrow{\mathrm{HBr}} \mathrm{B} \xrightarrow[\mathrm{KOH}_{(\mathrm{aq})}]{\Delta} \mathrm{C}$$
Given below are two statements:
Statement - I: High concentration of strong nucleophilic reagent with secondary alkyl halides which do not have bulky substituents will follow $$\mathrm{S}_{\mathrm{N}}{ }^2$$ mechanism.
Statement - II: A secondary alkyl halide when treated with a large excess of ethanol follows $$\mathrm{S}_{\mathrm{N}}{ }^1$$ mechanism.
In the light of the above statements, choose the most appropriate from the options given below:
Example of vinylic halide is :
The final product A, formed in the following multistep reaction sequence is :

Given below are two statements : one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A) : $$\mathrm{CH}_2=\mathrm{CH}-\mathrm{CH}_2-\mathrm{Cl}$$ is an example of allyl halide.
Reason (R) : Allyl halides are the compounds in which the halogen atom is attached to $$\mathrm{sp}^2$$ hybridised carbon atom.
In the light of the above statements, choose the most appropriate answer from the options given below :
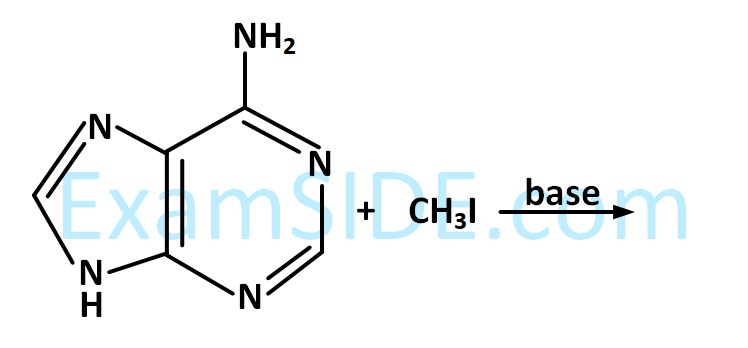
Alkyl halide is converted into alkyl isocyanide by reaction with
Given below are two statements : one is labelled as Assertion A and the other is labelled as Reason R :
Assertion A : Aryl halides cannot be prepared by replacement of hydroxyl group of phenol by halogen atom.
Reason R : Phenols react with halogen acids violently.
In the light of the above statements, choose the most appropriate from the options given below :
Identify B formed in the reaction.
$$\mathrm{Cl}-\left(\mathrm{CH}_2\right)_4-\mathrm{Cl} \xrightarrow{\text { excess } \mathrm{NH}_3} \mathrm{~A} \xrightarrow{\mathrm{NaOH}} \mathrm{B}+\mathrm{H}_2 \mathrm{O}+\mathrm{NaCl}$$
Which among the following halide/s will not show $$\mathrm{S_N 1}$$ reaction:
(A) $$\mathrm{H}_2 \mathrm{C}=\mathrm{CH}-\mathrm{CH}_2 \mathrm{Cl}$$
(B) $$\mathrm{CH}_3-\mathrm{CH}=\mathrm{CH}-\mathrm{Cl}$$
(C) 
(D) 
Choose the most appropriate answer from the options given below :
The correct statement regarding nucleophilic substitution reaction in a chiral alkyl halide is ;
In the following reaction 'X' is

Compound from the following that will not produce precipitate on reaction with $$\mathrm{AgNO}_{3}$$ is :

Find out the correct statement from the options given below for the above 2 reactions.
Identify the correct order of reactivity for the following pairs towards the respective mechanism

Choose the correct answer from the options given below:
The pair from the following pairs having both compounds with net non-zero dipole moment is
Major product '$$\mathrm{P}$$' formed in the following reaction is:

The correct order of reactivity of following haloarenes towards nucleophilic substitution with aqueous $$\mathrm{NaOH}$$ is :

Choose the correct answer from the options given below:
Choose the halogen which is most reactive towards $$\mathrm{S}_{\mathrm{N}} 1$$ reaction in the given compounds (A, B, C & D)

For the reaction

The correct statement is
The correct order of melting points of dichlorobenzenes is
Decreasing order towards SN 1 reaction for the following compounds is:

Match List I with List II
| List I | List II | ||
|---|---|---|---|
| A. |  |
I. | Fittig reaction |
| B. |  |
II. | Wurtz Fittig reaction |
| C. |  |
III. | Finkelstein reaction |
| D. | $$\mathrm{C_5H_5C1+NaI\to C_2H_5I+NaCl}$$ | IV. | Sandemyer reaction |
Choose the correct answer from the options given below :
Identify the correct order for the given property for following compounds.

Choose the correct answer from the option given below :
The isomeric deuterated bromide with molecular formula $$\mathrm{C_4H_8DBr}$$ having two chiral carbon atoms is
The compound which will have the lowest rate towards nucleophilic aromatic substitution on treatment with OH$$^-$$ is
Assertion A : Hydrolysis of an alkyl chloride is a slow reaction but in the presence of NaI, the rate of the hydrolysis increases.
Reason R : I$$^{-}$$ is a good nucleophile as well as a good leaving group.
In the light of the above statements, choose the correct answer from the options given below
Compound 'A' undergoes following sequence of reactions to give compound 'B'.
The correct structure and chirality of compound 'B' is
[where Et is $$\left.-\mathrm{C}_{2} \mathrm{H}_{5}\right]$$

Which among the following pairs of the structures will give different products on ozonolysis? (Consider the double bonds in the structures are rigid and not delocalized.)

Considering the above reactions, the compound 'A' and compound 'B' respectively are :
The major product in the given reaction is

Major product '$$\mathrm{B}$$' of the following reaction sequence is :


The stable carbocation formed in the above reaction is
Two isomers (A) and (B) with Molar mass 184 g/mol and elemental composition C, 52.2%; H, 4.9% and Br 42.9% gave benzoic acid and p-bromobenzoic acid, respectively on oxidation with KMnO4. Isomer 'A' is optically active and gives a pale yellow precipitate when warmed with alcoholic AgNO3. Isomer 'A' and 'B' are, respectively

In the given conversion the compound A is :

In the above reaction 'A' is
The major product (P) in the reaction

is
Which one of the following compounds is inactive towards SN1 reaction?
The major product of the following reaction is :

Halogenation of which one of the following will yield m-substituted product with respect to methyl group as a major product?
The major product in the reaction

In the given reaction sequence, the major product 'C' is :


The compounds B and C respectively are :-

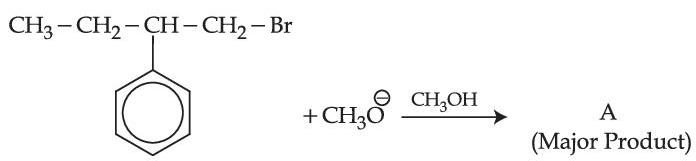


Consider the given reaction, the product A is :


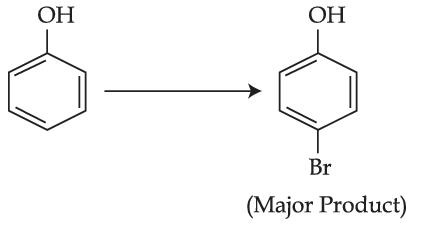
The given reaction can occur in the presence of :
(a) Bromine water
(b) Br2 in CS2, 273 K
(c) Br2/FeBr3
(d) Br2 in CHCl3, 273 K
Choose the correct answer from the options gien below :
Statement I : C2H5OH and AgCN both can generate nucleophile.
Statement II : KCN and AgCN both will generate nitrile nucleophile with all reaction conditions.
Choose the most appropriate option :
(A) AgCN/KCN
(B) RCOOAg/RCOOK
(C) AgNO2/KNO2
(D) AgI/KI
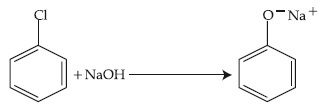
The above reaction requires which of the following reaction conditions?
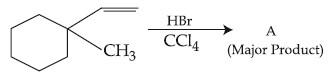
Product ''A'' in the above chemical reaction is :
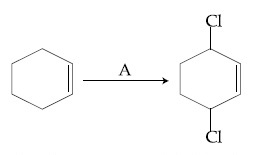
Identify the reagent(s) 'A' and condition(s) for the reaction
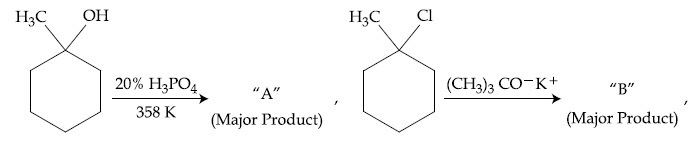
The products ''A'' and ''B'' formed in above reactions are :

Choose the correct answer from the options given below :

What is 'A'?

What is 'A'?



with excess $$\mathrm{Mg/Et_2O}$$ ($$\mathrm{Et=C_2H_5}$$) is
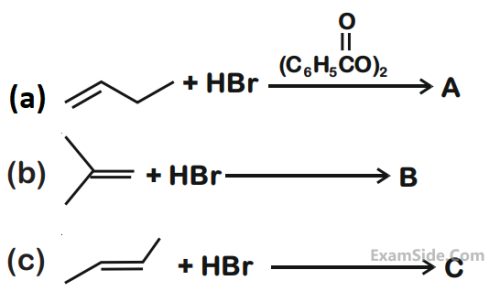
CH3CH = CHCH(CH3)2 $$\buildrel {HBr} \over \longrightarrow $$
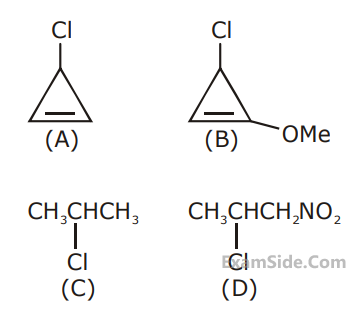



A student writes general characteristics based on the given mechanism as :
(a) The reaction is favoured by weak nucleophiles.
(b) R+ would be easily formed if the substituents are bulky.
(c) The reaction is accompanied by racemization.
(d) The reaction is favoured by non-polar solvents.
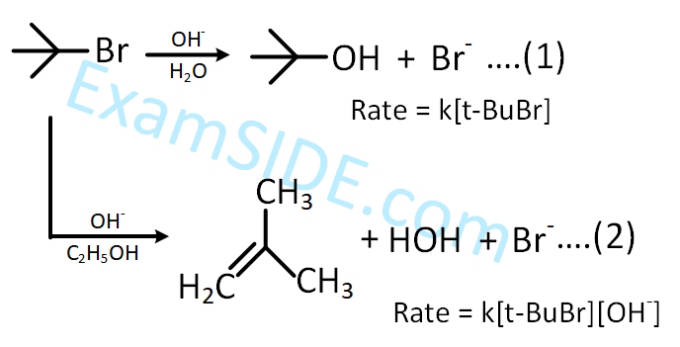
Which of the following statements is true?


Ks and Ke, are respectively, the rate constants for substitution and elimination and $$\mu = {{{k_s}} \over {{k_e}}}$$ the correct options is




Which of these reactions are possible ?
Assertion (A) : Vinyl halides do not undergo nucleophilic substitution easily.
Reason (R) : Even though the intermediate carbocation is stabilized by loosely held p-electrons, the cleavage is difficult because of strong bonding.




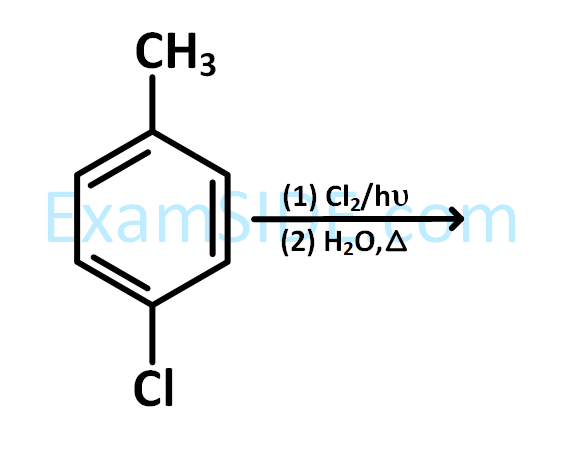
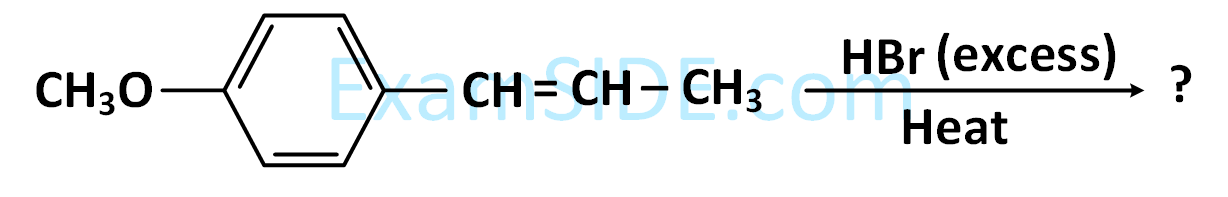
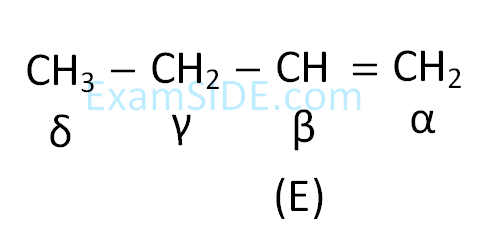






The compound I is :






CH3Cl, CH3CH 2Cl, (CH3)2CHCl and (CH3)3CCl is:
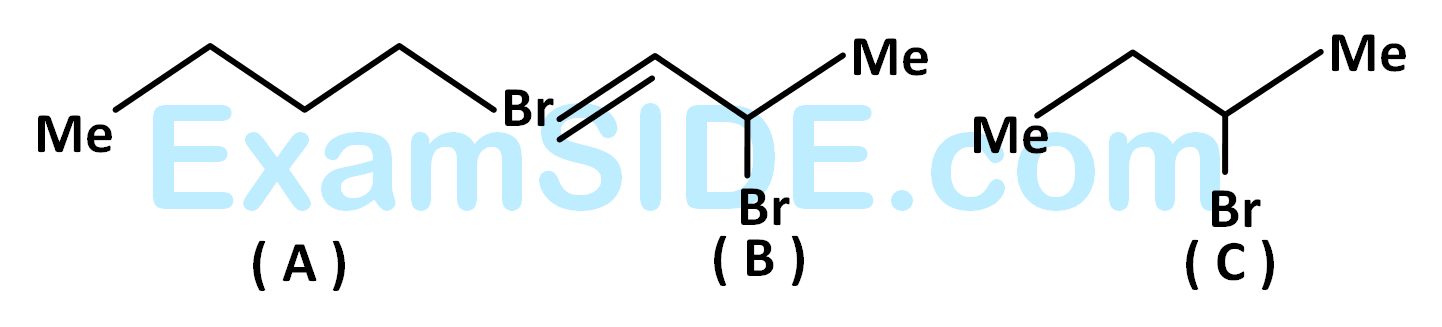
The correct order of $${S_N}1$$ reactive is
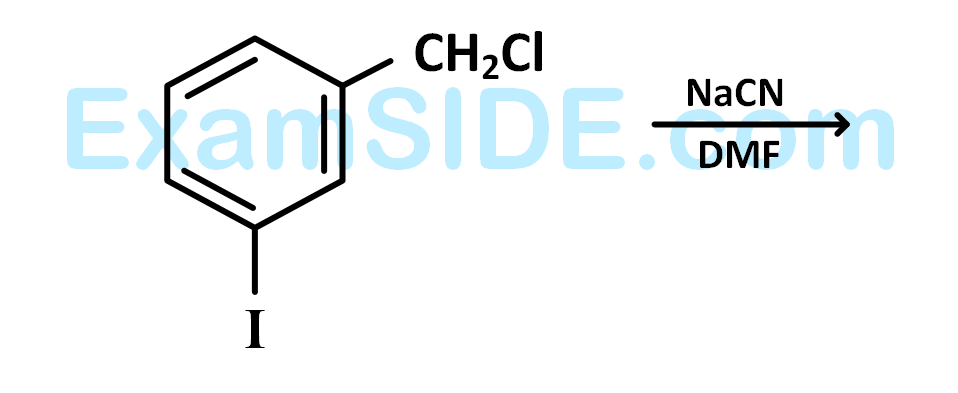
is
$${(CH)_3}C - Br\buildrel {{H_2}O} \over \longrightarrow {(CH)_3}C - OH$$
Numerical

Consider the above sequence of reactions. 151 g of 2-bromopentane is made to react. Yield of major product P is $80 \%$ whereas Q is $100 \%$.
Mass of product Q obtained is________ g.
(Given molar mass in $\mathrm{g} \mathrm{mol}^{-1} \mathrm{H}: 1, \mathrm{C}: 12, \mathrm{O}: 16, \mathrm{Br}: 80$ )
Consider the following sequence of reactions:

11.25 mg of chlorobenzene will produce ___________$\times 10^{-1} \mathrm{mg}$ of product B.
(Consider the reactions result in complete conversion.)
[Given molar mass of $\mathrm{C}, \mathrm{H}, \mathrm{O}, \mathrm{N}$ and Cl as $12,1,16,14$ and $35.5 \mathrm{~g} \mathrm{~mol}^{-1}$ respectively]

The ratio of number of oxygen atoms to bromine atoms in the product Q is _________ $$\times 10^{-1}$$.
The number of halobenzenes from the following that can be prepared by Sandmeyer's reaction is _________


The total number of hydrogen atoms in product A and product B is _________.
2-chlorobutane $$+\mathrm{Cl}_2 \rightarrow \mathrm{C}_4 \mathrm{H}_8 \mathrm{Cl}_2$$ (isomers)
Total number of optically active isomers shown by $$\mathrm{C}_4 \mathrm{H}_8 \mathrm{Cl}_2$$, obtained in the above reaction is _________.
Number of moles of AgCl formed in the following reaction is _____________

In the presence of sunlight, benzene reacts with Cl2 to give product, X. The number of hydrogens in X is _____________.
The major product of the following reaction contains ____________ bromine atom(s).


Consider the above reaction. The number of $$\pi$$ electrons present in the product 'P' is __________.
(i) forms aldehydes on ozonolysis followed by the hydrolysis.
(ii) when vaporized completely 1.53 g of A, gives 448 mL of vapour at STP.
The number of carbon atoms in a molecule of compound A is ___________.