Hydrocarbons · Chemistry · JEE Main
MCQ (Single Correct Answer)
The reactions which cannot be applied to prepare an alkene by elimination, are

Choose the correct answer from the options given below:
Given below are two statements:
Statement I : Ozonolysis followed by treatment with $\mathrm{Zn}, \mathrm{H}_2 \mathrm{O}$ of cis-2-butene gives ethanal.
Statement II : The product obtained by ozonolysis followed by treatment with $\mathrm{Zn}, \mathrm{H}_2 \mathrm{O}$ of 3, 6-dimethyloct-4-ene has no chiral carbon atom.
In the light of the above statements, choose the correct answer from the options given below
Predict the major product of the following reaction sequence:-

$$ \text { Which compound would give 3-methyl-6-oxoheptanal upon ozonolysis? } $$
Given below are two statements :
Statement (I) : Neopentane forms only one monosubstituted derivative.
Statement (II) : Melting point of neopentane is higher than n-pentane.
In the light of the above statements, choose the most appropriate answer from the options given below :
Match List - I with List - II.
| List - I (Reaction) |
List - II (Name of reaction) |
||
|---|---|---|---|
| (A) |  |
(I) | Lucas reaction |
| (B) | $$ \mathrm{ArN}_2^{+} \mathrm{X}^{-} \xrightarrow[\mathrm{HCl}]{\mathrm{Cu}} \mathrm{ArCl}+\mathrm{N}_2 \uparrow+\mathrm{CuX} $$ |
(II) | Finkelstein reaction |
| (C) | $$ \mathrm{C}_2 \mathrm{H}_5 \mathrm{Br}+\mathrm{NaI} \xrightarrow[\text { Acetone }]{\text { Dry }} \mathrm{C}_2 \mathrm{H}_5 \mathrm{I}+\mathrm{NaBr} $$ |
(III) | Fittig reaction |
| (D) | $$ \mathrm{CH}_3 \mathrm{C}(\mathrm{OH})\left(\mathrm{CH}_3\right) \mathrm{CH}_3 \xrightarrow[\mathrm{ZnCl}_2]{\mathrm{HCl}} \mathrm{CH}_3 \mathrm{C}(\mathrm{Cl})\left(\mathrm{CH}_3\right) \mathrm{CH}_3 $$ |
(IV) | Gatterman reaction |
$$ \text { Choose the correct answer from the options given below : } $$
Total number of sigma (σ) _______ and pi(π) ______ bonds respectively present in hex-1-en-4-yne are:
The product B formed in the following reaction sequence is:

Identify product [A], [B] and [C] in the following reaction sequence.
$\mathrm{CH}_3-\mathrm{C} \equiv \mathrm{CH} \xrightarrow[\mathrm{H}_2]{\mathrm{Pd} / \mathrm{C}}[\mathrm{A}] \xrightarrow[\text { (ii) } \mathrm{Zn}, \mathrm{H}_2 \mathrm{O}]{\text { (i) } \mathrm{O}_3}[\mathrm{~B}]+[\mathrm{C}]$
The product $(\mathrm{A})$ formed in the following reaction sequence is

Match List - I with List - II
| List - I (Isomers of $\mathrm{C}_{10} \mathrm{H}_{14}$ ) |
List - II (Ozonolysis product) |
||
|---|---|---|---|
| (A) |  |
(I) |  |
| (B) |  |
(II) |  |
| (C) |  |
(III) |  |
| (D) |  |
(IV) |  |
Choose the correct answer from the options given below :
Match the List - I with List - II
| List - I Name reaction |
List - II Product obtainable |
||
|---|---|---|---|
| (A) | Swarts reaction | (I) | Ethyl benzene |
| (B) | Sandmeyer's reaction | (II) | Ethyl iodide |
| (C) | Wurtz Fittig reaction | (III) | Cyanobenzene |
| (D) | Finkelstein reaction | (IV) | Ethyl fluoride |
Choose the correct answer from the options given below:
When sec-butylcyclohexane reacts with bromine in the presence of sunlight, the major product is :
The alkane from below having two secondary hydrogens is :
Given below are two statements :
Statement I : One mole of propyne reacts with excess of sodium to liberate half a mole of $\mathrm{H}_2$ gas.
Statement II : Four g of propyne reacts with $\mathrm{NaNH}_2$ to liberate $\mathrm{NH}_3$ gas which occupies 224 mL at STP.
In the light of the above statements, choose the most appropriate answer from the options given below :
The incorrect statement regarding ethyne is
Identify the product A and product B in the following set of reactions.

Given below are two statements :
Statement (I) : $$\mathrm{S}_{\mathrm{N}} 2$$ reactions are 'stereospecific', indicating that they result in the formation of only one stereo-isomer as the product.
Statement (II) : $$\mathrm{S}_{\mathrm{N}} 1$$ reactions generally result in formation of product as racemic mixtures.
In the light of the above statements, choose the correct answer from the options given below :
In the given compound, the number of 2$$^\circ$$ carbon atom/s is ________.


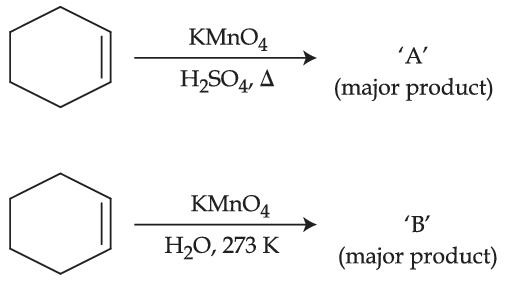
Consider the above chemical reaction. Product "A" is :
Given below are two statements :
Statement I : Nitration of benzene involves the following step -

Statement II : Use of Lewis base promotes the electrophilic substitution of benzene.
In the light of the above statements, choose the most appropriate answer from the options given below :

In the above chemical reaction sequence "$$\mathrm{A}$$" and "$$\mathrm{B}$$" respectively are


Acid D formed in above reaction is :
Major product of the following reaction is -

Products A and B formed in the following set of reactions are

Compound A formed in the following reaction reacts with B gives the product C. Find out A and B.

In the given reactions, identify the reagent A and reagent B.

Identify product A and product B :

The final product A formed in the following multistep reaction sequence is

The final product A, formed in the following reaction sequence is:



In the above reaction, left hand side and right hand side rings are named as '$$\mathrm{A}$$' and 'B' respectively. They undergo ring expansion. The correct statement for this process is:
The major product formed in the following reaction is


Choose the correct answer from the options given below :
Given below are two statements, one is labelled as Assertion A and the other is labelled as Reason R.

In the light of the above statements, choose the correct answer from the options given below:
Find out the major product from the following reaction.

In the following reaction, 'B' is

'$$\mathrm{X}$$' is :

But-2-yne is reacted separately with one mole of Hydrogen as shown below :

A. A is more soluble than B.
B. The boiling point & melting point of A are higher and lower than B respectively.
C. A is more polar than B because dipole moment of A is zero.
D. $$\mathrm{Br_2}$$ adds easily to B than A.
Identify the incorrect statements from the options given below :
Choose the correct set of reagents for the following conversion.
trans $$\left(\mathrm{Ph}-\mathrm{CH}=\mathrm{CH}-\mathrm{CH}_{3}\right) \rightarrow \operatorname{cis}\left(\mathrm{Ph}-\mathrm{CH}=\mathrm{CH}-\mathrm{CH}_{3}\right)$$
The major products 'A' and 'B', respectively, are

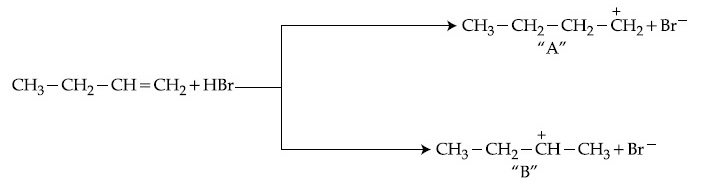
The one giving maximum number of isomeric alkenes on dehydrohalogenation reaction is (excluding rearrangement)

The correct sequence of reagents for the preparation of Q and R is :
Find out the major products from the following reactions.

In the following given reaction, 'A' is

$$ \stackrel{\bullet}{\mathrm{Cl}}+\mathrm{CH}_{4} \rightarrow \mathrm{A}+\mathrm{B} $$
A and B in the above atmospheric reaction step are :
Major product of the following reaction is

A compound ‘$$\mathrm{A}$$’ on reaction with ‘$$\mathrm{X}$$’ and ‘$$\mathrm{Y}$$’ produces the same major product but different by product '$$a$$' and '$$b^{\prime}$$. Oxidation of '$$a$$' gives a substance produced by ants.

'X' and 'Y' respectively are
Two isomers 'A' and 'B' with molecular formula C4H8 give different products on oxidation with KMnO4 in acidic medium. Isomer 'A' on reaction with KMnO4/H+ results in effervescence of a gas and gives ketone. The compound 'A' is
What will be the major product of following sequence of reactions?

Product 'A' of following sequence of reactions is

The major product in the following reaction

The product formed in the following reaction.

Given below are two statements :
Statement I : The presence of weaker $$\pi$$-bonds make alkenes less stable than alkanes.
Statement II : The strength of the double bond is greater than that of carbon-carbon single bond.
In the light of the above statements, choose the correct answer from the options given below :
Which of the following reagents / reactions will convert 'A' to 'B' ?

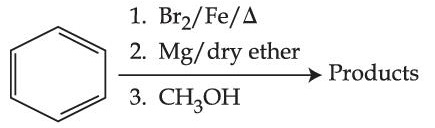

CH4 + I2 $$\mathrel{\mathop{\kern0pt\rightleftharpoons} \limits_{{\mathop{\rm Re}\nolimits} versible}^{hv}} $$ CH3 $$-$$ I + HI
Compound 'A' also yields compound 'B' an ozonolysis. Compound 'A' is :

Major product P of above reaction, is :
For above chemical reactions, identify the correct statement from the following :
List - I (Chemicals)
(a) Alcoholic potassium hydroxide
(b) Pd/BaSO4
(c) BHC (Benzene hexachloride)
(d) Polyacetylene
List - II (Use / Preparation / Constituent)
(i) Electrodes in batteries
(ii) Obtained by addition reaction
(iii) Used for $$\beta$$ - elimination reaction
(iv) Lindlar's catalyst
Choose the most appropriate match :
Statement I : 2-methylbutane on oxidation with KMnO4 gives 2-methylbutan-2-ol.
Statement II : n-alkanes can be easily oxidised to corresponding alcohols with KMnO4.
Choose the correct option :













$${\left( {C{H_3}} \right)_3}CCH\left( {OH} \right)C{H_3}\buildrel {conc.\,\,\,{H_2}S{O_4}} \over \longrightarrow $$
$${\left( {C{H_3}} \right)_2}CHCH\left( {Br} \right)C{H_3}\buildrel {alc.\,\,KOH} \over \longrightarrow $$
$${\left( {C{H_3}} \right)_2}{\rm{ }}CHCH\left( {Br} \right)C{H_3}\buildrel {{{(C{H_3})}_3}{O^\Theta }{K^ \oplus }} \over \longrightarrow $$
$${\left( {C{H_3}} \right)_2}\mathop C\limits_{\mathop |\limits_{OH} } - C{H_2} - CHO\buildrel \Delta \over \longrightarrow $$
Which of these reaction(s) will not produce Saytzeff product ?
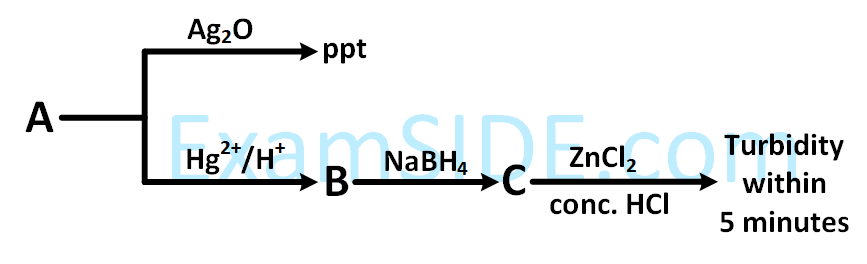
'A' is :
H3C–CH=CH2 $$\buildrel {C{l_2}/{H_2}O} \over \longrightarrow $$



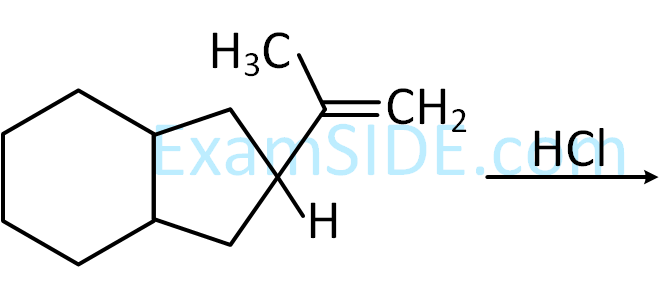
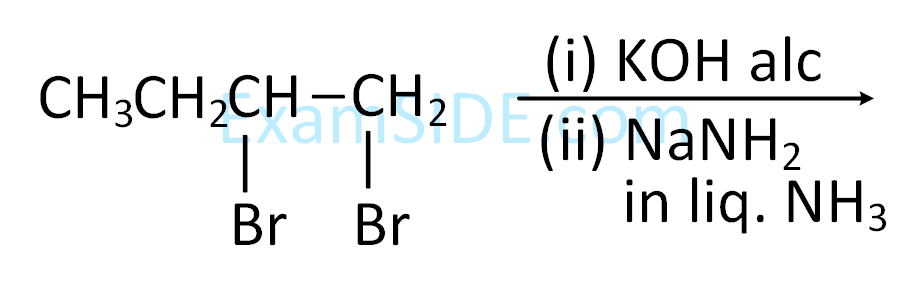
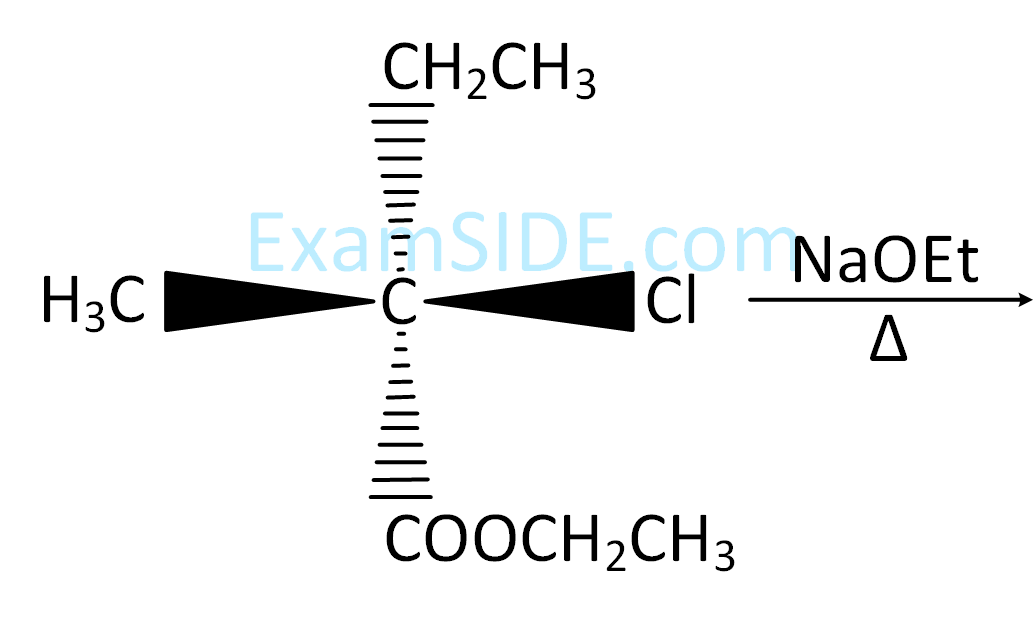

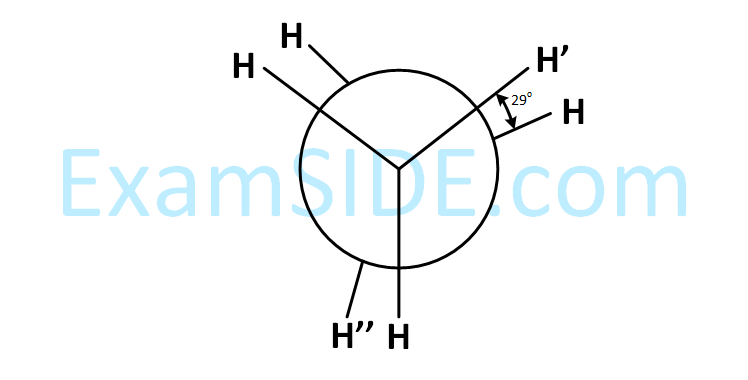
CH $$ \equiv $$ CH, CH3–C $$ \equiv $$ CH and CH2 = CH2 is as follows


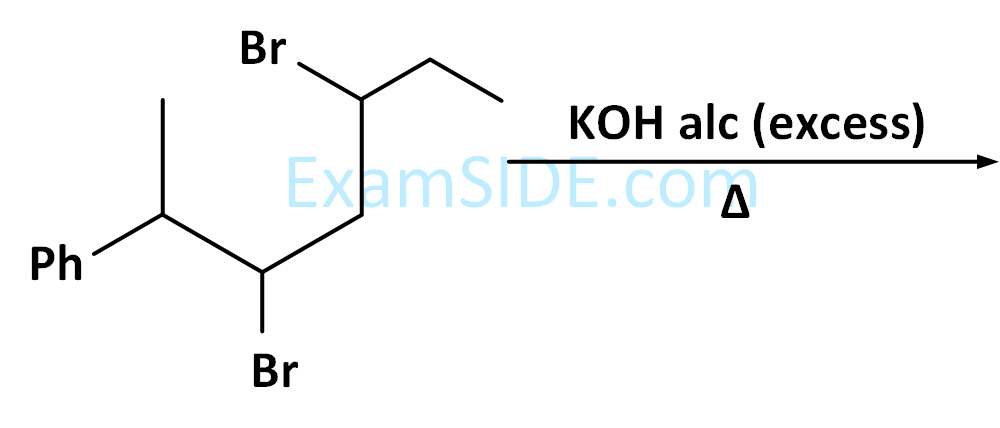




X is:
$$\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,$$ $${C_6}{H_5}C{H_2}CH\left( {OH} \right)CH{\left( {C{H_3}} \right)_2}\buildrel {conc.{H_2}S{O_4}} \over \longrightarrow \,?$$
CH3 - CH = CH - CH3 $$\buildrel {{O_3}} \over \longrightarrow $$ A $$\mathrel{\mathop{\kern0pt\longrightarrow} \limits_{Zn}^{H{}_2O}} $$ B
The compound B is
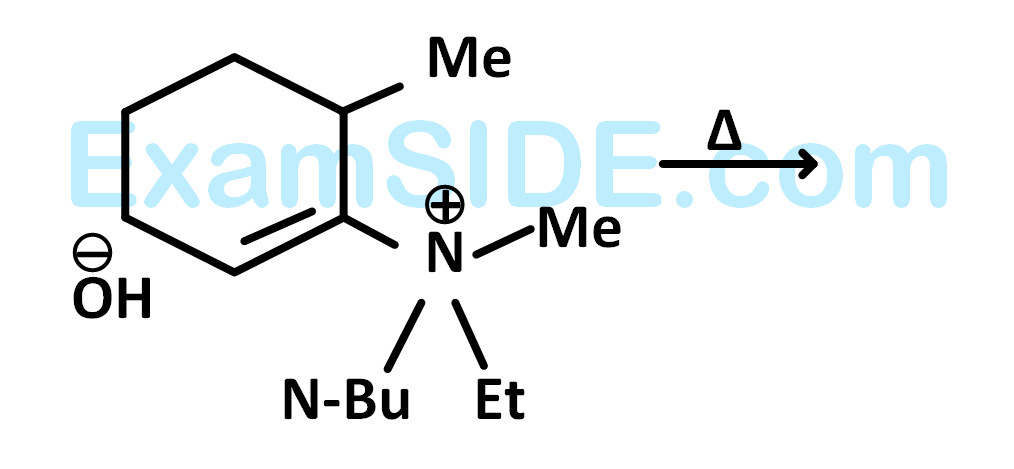
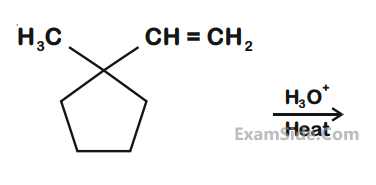
The alkene formed as a major product in the above elimination reaction is
Numerical
Isomeric hydrocarbons → negative Baeyer’s test
(Molecular formula C9H12)
The total number of isomers from above with four different non-aliphatic substitution sites is -
The sum of sigma (σ) and pi (π) bonds in Hex-1,3-dien-5-yne is ________.
The compound with molecular formula $\mathrm{C}_6 \mathrm{H}_6$, which gives only one monobromo derivative and takes up four moles of hydrogen per mole for complete hydrogenation has _________ $\pi$ electrons.
Major product B of the following reaction has ________ $$\pi$$-bond.

The major product of the following reaction is P.
$$\mathrm{CH}_3 \mathrm{C}\equiv\mathrm{C}-\mathrm{CH}_3$$ $$\xrightarrow[\substack{\text { (ii) dil. } \mathrm{KMnO}_4 \\ 273 \mathrm{~K}}]{\text { (i) } \mathrm{Na} \text { /liq. } \mathrm{NH}_3}$$ 'P'
Number of oxygen atoms present in product '$$\mathrm{P}$$' is _______. (nearest integer)
The major products from the following reaction sequence are product A and product B.

The total sum of $$\pi$$ electrons in product A and product B are __________ (nearest integer)

Consider the given reaction. The total number of oxygen atom/s present per molecule of the product $$(\mathrm{P})$$ is _________.
For the given reaction

The total number of possible products formed by tertiary carbocation of A is ____________.
The number of possible isomeric products formed when 3-chloro-1-butene reacts with $$\mathrm{HCl}$$ through carbocation formation is __________.
Molar mass of the hydrocarbon (X) which on ozonolysis consumes one mole of $$\mathrm{O}_{3}$$ per mole of $$(\mathrm{X})$$ and gives one mole each of ethanal and propanone is _________ $$\mathrm{g}~ \mathrm{mol}^{-1}$$
(Molar mass of $$\mathrm{C}: 12 \mathrm{~g} \mathrm{~mol}^{-1}, \mathrm{H}: 1 \mathrm{~g} \mathrm{~mol}^{-1}$$ )
Number of bromo derivatives obtained on treating ethane with excess of $$\mathrm{Br}_{2}$$ in diffused sunlight is ___________
17 mg of a hydrocarbon (M.F. $$\mathrm{C_{10}H_{16}}$$) takes up 8.40 mL of the H$$_2$$ gas measured at 0$$^\circ$$C and 760 mm of Hg. Ozonolysis of the same hydrocarbon yields

The number of double bond/s present in the hydrocarbon is ___________.
Maximum number of isomeric monochloro derivatives which can be obtained from 2,2,5,5-tetramethylhexane by chlorination is _____________
The major product 'A' of the following given reaction has _____________ sp2 hybridized carbon atoms.


Consider the above chemical reaction. The total number of stereoisomers possible for Product 'P' is _____________.
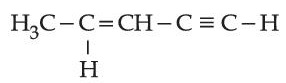
is _______________.

The number of sp2 hybridized carbon atom(s) present in the product is ________.

 (A is a lowest molecular weight alkyne)
(A is a lowest molecular weight alkyne)