Aldehydes, Ketones and Carboxylic Acids · Chemistry · JEE Main
MCQ (Single Correct Answer)
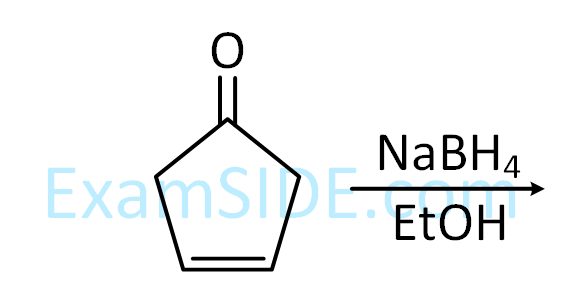
 undergoes intramolecular aldol condensation, the major product formed is :
undergoes intramolecular aldol condensation, the major product formed is :
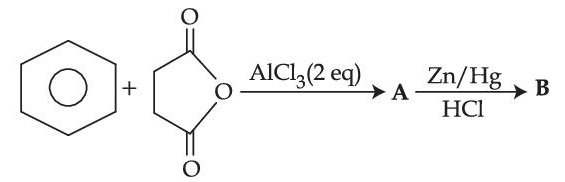
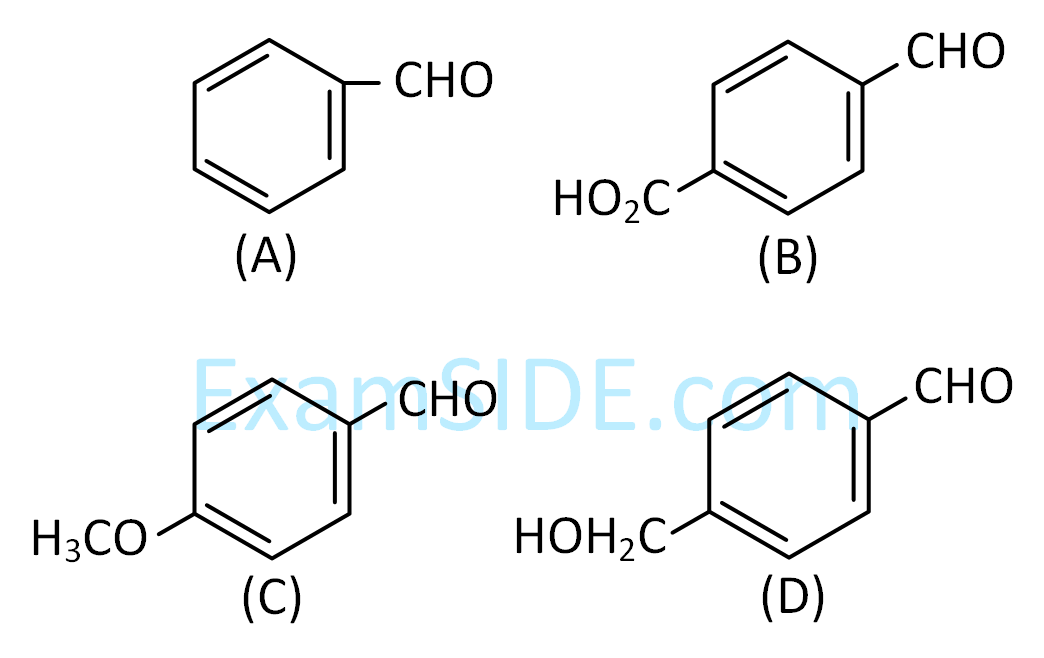
Choose the correct option for structures of $A$ and $B$, respectively
Which among the following compounds give yellow solid when reacted with $\mathrm{NaOI} / \mathrm{NaOH}$ ?

Choose the correct answer from the options given below :
Aldol condensation is a popular and classical method to prepare $\alpha, \beta$-unsaturated carbonyl compounds. This reaction can be both intermolecular and intramolecular. Predict which one of the following is not a product of intramolecular aldol condensation?
An organic compound $(\mathrm{X})$ with molecular formula $\mathrm{C}_3 \mathrm{H}_6 \mathrm{O}$ is not readily oxidised. On reduction it gives $\mathrm{C}_3 \mathrm{H}_8 \mathrm{O}(\mathrm{Y})$ which reacts with HBr to give a bromide $(\mathrm{Z})$ which is converted to Grignard reagent. This Grignard reagent on reaction with $(\mathrm{X})$ followed by hydrolysis give 2,3-dimethylbutan-2-ol. Compounds $(\mathrm{X}),(\mathrm{Y})$ and $(\mathrm{Z})$ respectively are:
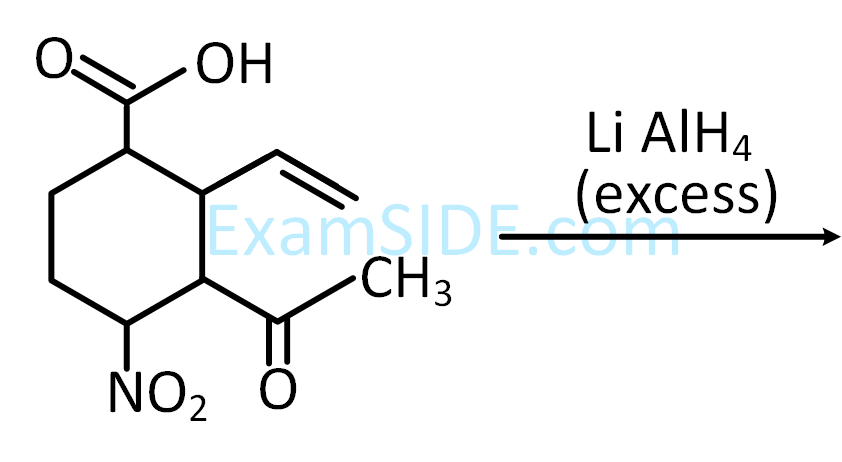
$$ \text {The major product }(\mathrm{P}) \text { in the following reaction is : } $$

Number of molecules from below which cannot give iodoform reaction is :
Ethanol, Isopropyl alcohol, Bromoacetone, 2-Butanol, 2-Butanone, Butanal, 2-Pentanone, 3-Pentanone, Pentanal and 3-Pentanol.
Consider the following reactions. From these reactions which reaction will give carboxylic acid as a major product ?
(A) $\quad \mathrm{R}-\mathrm{C} \equiv \mathrm{N} \xrightarrow[\text { mild condition }]{\text { (i) } \stackrel{+}{\mathrm{H}} / \mathrm{H}_2 \mathrm{O}}$
(B) $\quad \mathrm{R}-\mathrm{MgX} \xrightarrow[\text { (ii) } \mathrm{H}_3 \mathrm{O}^{+}]{\text {(i) } \mathrm{CO}_2}$
(C) $\mathrm{R}-\mathrm{C} \equiv \mathrm{N} \xrightarrow[\text { (ii) } \mathrm{H}_3 \mathrm{O}^{+}]{\text {(i) } \mathrm{SnCl}_2 / \mathrm{HCl}}$
(D) $\quad \mathrm{R} \cdot \mathrm{CH}_2 \cdot \mathrm{OH} \xrightarrow{\mathrm{PCC}}$
(E) 
Choose the correct answer from the options given below :
Given below are two statements:
Statement I : Vanilin  will react with NaOH and also with Tollen's reagent.
will react with NaOH and also with Tollen's reagent.
Statement II : Vanilin  will undergo self aldol condensation very easily.
will undergo self aldol condensation very easily.
In the light of the above statements, choose the most appropriate answer from the options given below :
An optically active alkyl halide $\mathrm{C}_4 \mathrm{H}_9 \mathrm{Br}[\mathrm{A}]$ reacts with hot KOH dissolved in ethanol and forms alkene $[B]$ as major product which reacts with bromine to give dibromide $[C]$. The compound [C] is converted into a gas [D] upon reacting with alcoholic $\mathrm{NaNH}_2$. During hydration 18 gram of water is added to 1 mole of gas [D] on warming with mercuric sulphate and dilute acid at 333 K to form compound [E]. The IUPAC name of compound [ E ] is :
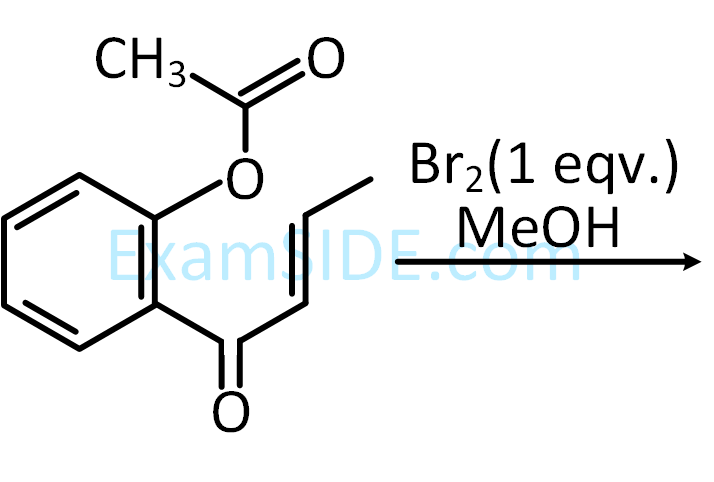
The product (P) formed in the following reaction is :

The total number of compounds from below when treated with hot KMnO4, giving benzoic acid is:


Both acetaldehyde and acetone (individually) undergo which of the following reactions?
A. Iodoform Reaction
B. Cannizaro Reaction
C. Aldol Condensation
D. Tollen's Test
E. Clemmensen Reduction
Choose the correct answer from the options given below:
The compounds that produce $\mathrm{CO}_2$ with aqueous $\mathrm{NaHCO}_3$ solution are:

Choose the correct answer from the options given below:
Match List - I with List - II.
| List - I | List - II | ||
|---|---|---|---|
| (A) |  |
(I) | Etard reaction |
| (B) |  |
(II) | Gatterman-Koch reaction |
| (C) |  |
(III) | Rosenmund reduction |
| (D) |  |
(IV) | Stephen reaction |
Choose the correct answer from the options given below :
Which of the following arrangements with respect to their reactivity in nucleophilic addition reaction is correct?
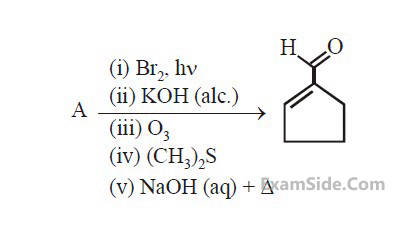
Aman has been asked to synthesise the molecule  He thought of preparing the molecule using an aldol condensation reaction. He found a few cyclic alkenes in his laboratory. He thought of performing ozonolysis reaction on alkene to produce a dicarbonyl compound followed by aldol reaction to prepare " $x$ ". Predict the suitable alkene that can lead to the formation of " $x$ ".
He thought of preparing the molecule using an aldol condensation reaction. He found a few cyclic alkenes in his laboratory. He thought of performing ozonolysis reaction on alkene to produce a dicarbonyl compound followed by aldol reaction to prepare " $x$ ". Predict the suitable alkene that can lead to the formation of " $x$ ".
Given below are two statements:
Consider the following reaction

Statement (I): In the case of formaldehyde  is about 2280, due to small substituents, hydration is faster.
is about 2280, due to small substituents, hydration is faster.
Statement (II) : In the case of trichloro acetaldehyde  is about 2000 due to $-$I effect of $-$Cl .
is about 2000 due to $-$I effect of $-$Cl .
In the light of the above statements, choose the correct answer from the options given below :
The major product of the following reaction is :
$\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}=\mathrm{O} \xrightarrow[\text { reflux }]{\substack{\text { excess } \mathrm{HCHO} \\ \text { alkali }}}$ ?

Residue (A) + HCl (dil) $\rightarrow$ Compound (B)
Structure of residue (A) and compound (B) formed respectively is :
The compounds which give positive Fehling's test are :

Choose the correct answer from the options given below :

In the above reaction product 'P' is
Which of the following compounds will give silver mirror with ammoniacal silver nitrate?
A. Formic acid
B. Formaldehyde
C. Benzaldehyde
D. Acetone
Choose the correct answer from the options given below :
Identify major product "X" formed in the following reaction :


What is the structure of C?
Identify the product (P) in the following reaction:

Consider the given reaction, identify the major product P.

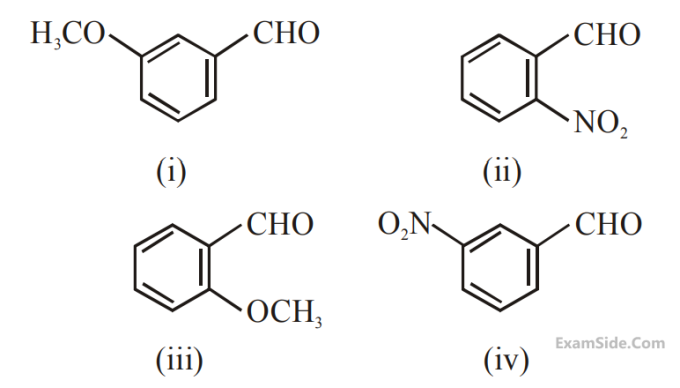
Which among the following aldehydes is most reactive towards nucleophilic addition reactions?
Identify A and B in the given chemical reaction sequence :

Consider the given chemical reaction :

Product "A" is :
Identify 'A' in the following reaction :

Given below are two statements :
Statements I : Acidity of $$\alpha$$-hydrogens of aldehydes and ketones is responsible for Aldol reaction.
Statement II : Reaction between benzaldehyde and ethanal will NOT give Cross - Aldol product.
In the light of the above statements, choose the most appropriate answer from the options given below :
Identify the product in the following reaction:


| List I (Reactions) | List II (Reagents) |
|---|---|
(A)  |
(I) CH3MgBr, H2O |
| (B) C6H5COC6H5 ⟶ C6H5CH=C6H5 | (II) Zn(Hg) and conc. HCl |
| (C) C6H5CHO ⟶ C6H5CH(OH)CH3 | (III) NaBH4, H+ |
(D)  |
(IV) DIBAL-H, H2O |
Choose the correct answer from the options given below :
Identify major product 'P' formed in the following reaction.

Identify the name reaction.

Salicylaldehyde is synthesized from phenol, when reacted with
m-chlorobenzaldehyde on treatment with 50% KOH solution yields :

This reduction reaction is known as:
Structure of 4-Methylpent-2-enal is :
Identify the reagents used for the following conversion

The molecular formula of second homologue in the homologous series of mono carboxylic acids is
Highest enol content will be shown by:

In the above conversion the correct sequence of reagents to be added is
In the reaction given below

'A' is

The two products formed in above reaction are -

'A' in the above reaction is:
In the following reaction

'A' is
The major product 'P' formed in the following sequence of reactions is

Given below are two statements, one is labelled as Assertion A and the other is labelled as Reason R.
Assertion A : A solution of the product obtained by heating a mole of glycine with a mole of chlorine in presence of red phosphorous generates chiral carbon atom.
Reason R : A molecule with 2 chiral carbons is always optically active.
In the light of the above statements, choose the correct answer from the options given below:

'A' and 'B' in the above reactions are:
The major product 'P' formed in the given reaction is

The major product formed in the following reaction is:

Match List I with List II:
| LIST I (Reagents used) | LIST II (Compound with Functional group detected) | ||
|---|---|---|---|
| A. | Alkaline solution of copper sulphate and sodium citrate | I. |  |
| B. | Neutral $$\mathrm{FeCl_3}$$ | II. |  |
| C. | Alkaline chloroform solution | III. |  |
| D. | Potassium iodide and sodium hypochlorite | IV. |  |
Choose the correct answer from the options given below:
In a reaction,

reagents 'X' and 'Y' respectively are :
The structures of major products A, B and C in the following reaction are sequence.

Consider the following reaction

The correct statement for product B is. It is
Assertion A:
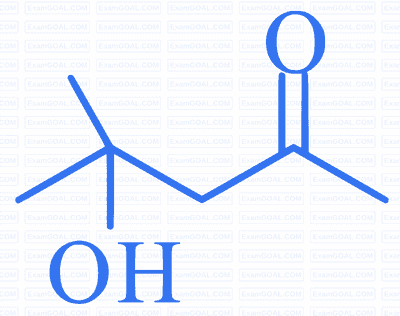 can be easily reduced using $\mathrm{Zn}-\mathrm{Hg} / \mathrm{HCl}$ to
can be easily reduced using $\mathrm{Zn}-\mathrm{Hg} / \mathrm{HCl}$ to 
Reason $\mathrm{R}: \mathrm{Zn}-\mathrm{Hg} / \mathrm{HCl}$ is used to reduce carbonyl group to $-\mathrm{CH}_{2}-$ group.
In the light of the above statements, choose the correct answer from the options given below:
What is the correct order of acidity of the protons marked $$\mathrm{A}-\mathrm{D}$$ in the given compounds ?

Which of the following compounds would give the following set of qualitative analysis ?
(i) Fehling's Test : Positive
(ii) Na fusion extract upon treatment with sodium nitroprusside gives a blood red colour but not prussian blue.
Find out the major products from the following reaction sequence.

'A' in the given reaction is

Identify the product formed (A and E)

'R' formed in the following sequence of reactions is :

Compound (X) undergoes following sequence of reactions to give the Lactone (Y).

Correct structure of $$\gamma$$-methylcyclohexane carbaldehyde is

Consider the above reaction sequence, the Product 'C' is :

Find out the major product for the above reaction.
The structure of A in the given reaction is :

Match List - I with List - II.
| List - I | List - II | ||
|---|---|---|---|
| (A) |  |
(I) | Gatterman Koch reaction |
| (B) | $$C{H_3} - CN\mathrel{\mathop{\kern0pt\longrightarrow} \limits_{{H_3}{O^ + }}^{SnC{l_2}/HCl}} C{H_3} - CHO$$ |
(II) | Etard reaction |
| (C) |  |
(III) | Stephen reaction |
| (D) |  |
(IV) | Rosenmund reaction |
Choose the correct answer from the options given below :

Consider the above reaction and predict the major product.
The products formed in the following reaction, A and B are

What is the major product of the following reaction?

$$C{H_3} - C{H_2} - CN\mathrel{\mathop{\kern0pt\longrightarrow} \limits_{Ether}^{C{H_3}MgBr}} A\buildrel {{H_3}{O^ + }} \over \longrightarrow B\mathrel{\mathop{\kern0pt\longrightarrow} \limits_{HCl}^{Zn - Hg}} C$$
The correct structure of C is
Which one of the following reactions does not represent correct combination of substrate and product under the given conditions?
Consider the given chemical reaction

Identify the product P.
Choose the reaction which is not possible:
Which among the following will be the major product of the given reaction?

Given below are two statements :
Statement I : The esterification of carboxylic acid with an alcohol is a nucleophilic acyl substitution.
Statement II : Electron withdrawing groups in the carboxylic acid will increase the rate of esterification reaction.
Choose the most appropriate option :
Isobutyraldehyde on reaction with formaldehyde and K2CO3 gives compound 'A'. Compound 'A' reacts with KCN and yields compound 'B', which on hydrolysis gives a stable compound 'C'. The compound 'C' is
The correct structure of product 'A' formed in the following reaction.

is
'A' and 'B' respectively are:

Which of the following reactions will yield benzaldehyde as a product?




Oxidation of toluene to benzaldehyde can be easily carried out with which of the following reagents?
The reagent, from the following, which converts benzoic acid to benzaldehyde in one step is

The final product 'A' in the following reaction sequence

(C7H5O2)2 $$\buildrel {hv} \over \longrightarrow $$ [X] $$\to$$ 2C6H5 + 2CO2
Consider the above reaction and identify the intermediate 'X'
Which will have the highest enol content?
Which of the following conditions or reaction sequence will NOT give acetophenone as the major product?
Two statements are given below :
Statement I : The melting point of monocarboxylic acid with even number of carbon atoms is higher than that of with odd number of carbon atoms acid immediately below and above it in the series.
Statement II : The solubility of monocarboxylic acids in water decreases with increase in molar mass.
Choose the most appropriate option :

Statement I : The nucleophilic addition of sodium hydrogen sulphite to an aldehyde or a ketone involves proton transfer to form a stable ion.
Statement II : The nucleophilic addition of hydrogen cyanide to an aldehyde or a ketone yields amine as final product.
In the light of the above statements, choose the most appropriate answer from the options given below :
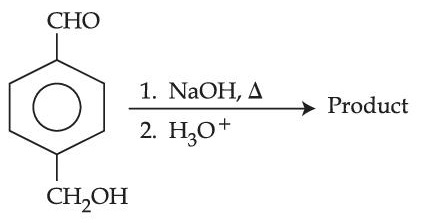
The compound which is not formed as a product in the reaction is a :

Statement I : Ethyl pent-4-yn-oate on reaction with CH3MgBr gives a 3$$^\circ$$-alcohol.
Statement II : In this reaction one mole of ethyl pent-4-yn-oate utilizes two moles of CH3MgBr.
In the light of the above statements, choose the most appropriate answer from the options given below :



Maleic anhydride can prepared by :

[where $$Et \Rightarrow - {C_2}{H_5}{}^tBu \Rightarrow {(C{H_3})_3}C - $$]
Consider the above reaction sequence, Product "A" and Product "B" formed respectively are :

Consider the given reaction, the product 'X' is :


The correct order of their reactivity towards hydrolysis at room temperature is :
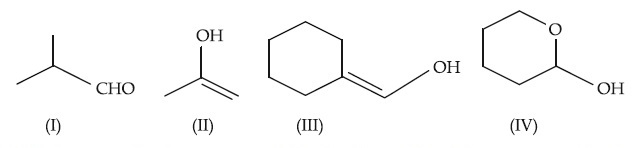
Which among the above compound/s does/do not form Silver mirror when treated with Tollen's reagent?

Consider the above reaction, the product 'X' and 'Y' respectively are :

Consider the above chemical reaction and identify product "A"

Considering the above chemical reaction, identify the product "X" :
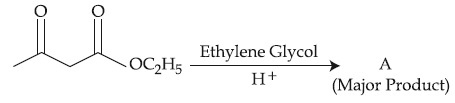
The product ''A'' in the above reaction is :
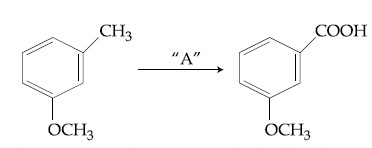
In the above reaction, the reagent ''A'' is :
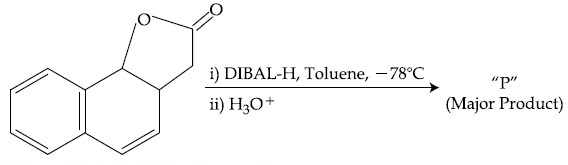
The product ''P'' in the above reaction is :
Reason R : Enol form of acetyl acetone is stabilized by intramolecular hydrogen bonding, which is not possible in enol form of acetone.
Choose the correct statement :


Considering the above reaction, the major product among the following is :





Which of the following reagent is suitable for the preparation of the product in the above reaction?
| List-I | List-II | ||
|---|---|---|---|
| (a) |  |
(i) | $$\mathrm{Br}_{2} / \mathrm{NaOH}$$ |
| (b) |  |
(ii) | $$\mathrm{H}_{2} / \mathrm{Pd}-\mathrm{BaSO}_{4}$$ |
| (c) |  |
(iii) | $$\mathrm{Zn}(\mathrm{Hg}) / \mathrm{H}$$ Conc. $$\mathrm{HCl}$$ |
| (d) |  |
(iv) | $$\mathrm{Cl}_{2}$$ Red $$\mathrm{P}, \mathrm{H}_{2} \mathrm{O}$$ |
Choose the correct answer from the options given below :
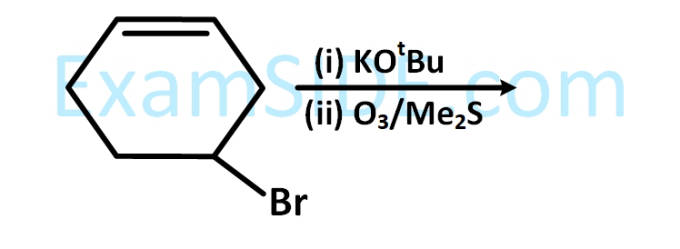
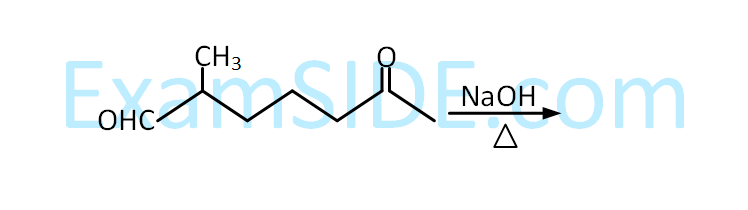
$$ \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_3 \stackrel{?}{\longrightarrow} \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CHO} $$
| Item-I | Item-II |
|---|---|
| (I) Benzene | (P) HCl and SnCl2, H3O+ |
| (II) Benzonitrile | (Q) H2, Pd-BaSO4, S and quinoline |
| (III) Benzoyl Chloride | (R) CO, HCl and AlCl3 |


'A' is :
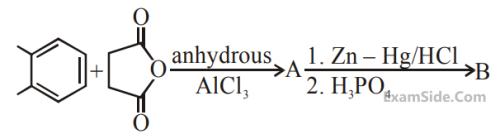
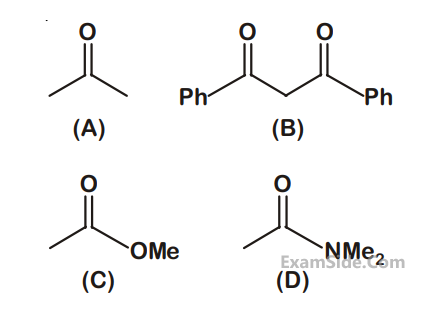


(a) (B) is more likely to be crystalline than (A)
(b) (B) has higher boiling point than (A)
(c) (B) dissolves more readily than (A) in water
Identify the correct option from below :

Propanal, Benzaldehyde, Propanone, Butanone
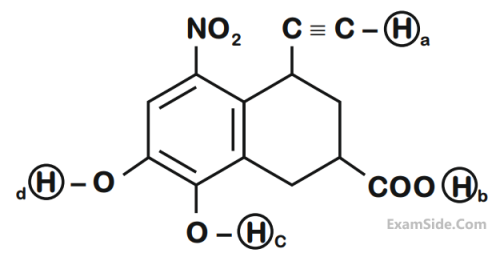
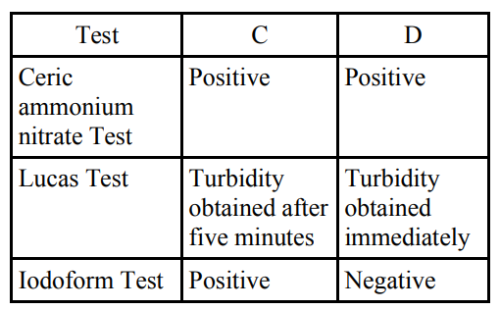
C and D respectively are :

 B(3 - oxo - hexanedicarboxylic acid) X will be :-
B(3 - oxo - hexanedicarboxylic acid) X will be :-










Aldehyde + Alcohol $$\buildrel {HCl} \over \longrightarrow $$ Acetal
| Aldehyde | Alcohol | ||
|---|---|---|---|
| HCHO | tBuOH | ||
| CH3CHO | MeOH |
The best combination is




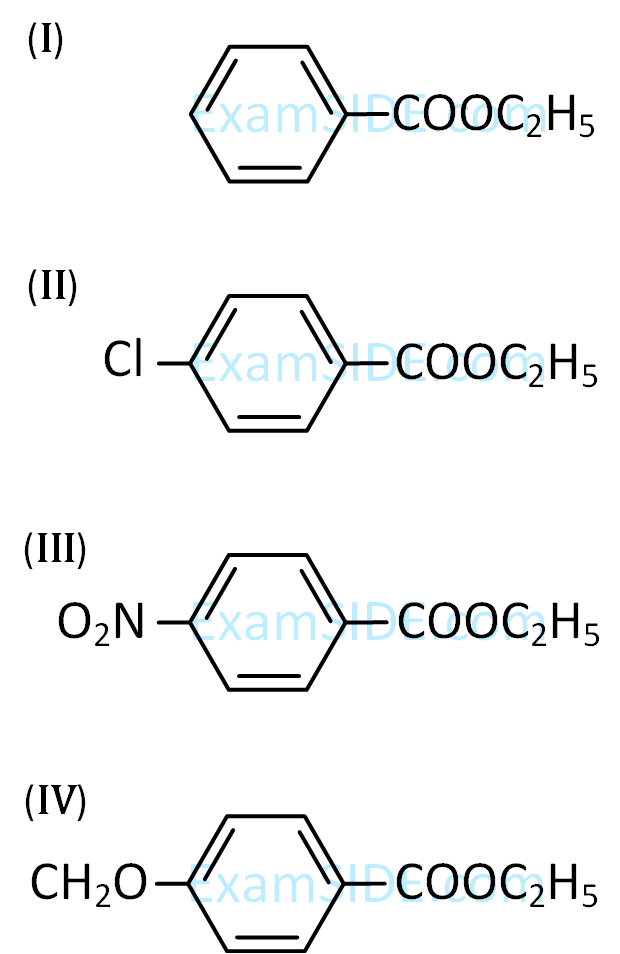


| Item - I | Item - II | ||
|---|---|---|---|
| (A) | Benzaldehyde | (P) | Mobile phase |
| (B) | Alumina | (Q) | Adsorbent |
| (C) | Acetonitrile | (R) | Adsorbate |









The product C is

The major product $$C$$ would be

the product C is:

2PhCHO $$\buildrel {\mathop {:OH}\limits^{\left( - \right)} } \over \longrightarrow $$ PhCH2OH + $$PhC\mathop O\limits^{..} $$2(-)
the slowest step is :
(A) HCHO
(B) CH3COCH3
(C) PhCOCH3
(D) PhCOPh
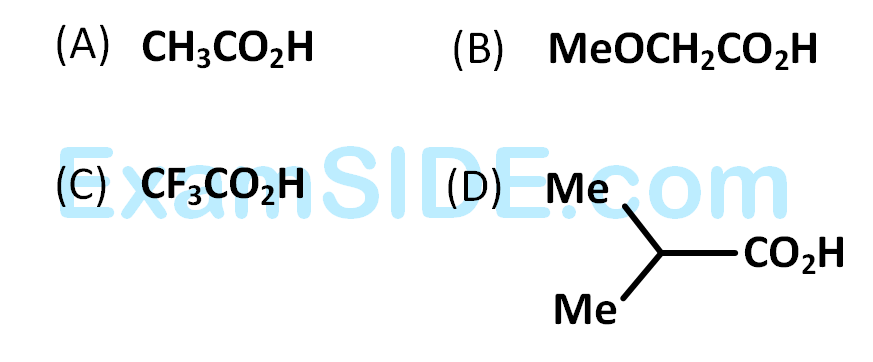

What is B?

Numerical
Identify the structure of the final product (D) in the following sequence of the reactions :

Total number of $\mathrm{sp}^2$ hybridised carbon atoms in product D is ____________.
A compound ' $\mathrm{X}^{\prime}$ absorbs 2 moles of hydrogen and ' X ' upon oxidation with $\mathrm{KMnO}_4 \mid \mathrm{H}^{+}$ gives

The total number of $\sigma$ bonds present in the compound ' $X^{\prime}$ ' is __________.
Two moles of benzaldehyde and one mole of acetone under alkaline conditions using aqueous $$\mathrm{NaOH}$$ after heating gives $$x$$ as the major product. The number of $$\pi$$ bonds in the product $$x$$ is ______.
The product C in the following sequence of reactions has ________ $$\pi$$ bonds.

In the Claisen-Schmidt reaction to prepare $$351 \mathrm{~g}$$ of dibenzalacetone using $$87 \mathrm{~g}$$ of acetone, the amount of benzaldehyde required is _________ g. (Nearest integer)
Vanillin compound obtained from vanilla beans, has total sum of oxygen atoms and $$\pi$$ electrons is __________.
The product of the following reaction is P.

The number of hydroxyl groups present in the product P is ________.
The total number of 'Sigma' and 'Pi' bonds in 2-formylhex-4-enoic acid is _________.
From the compounds given below, number of compounds which give positive Fehling's test is _________.
Benzaldehyde, Acetaldehyde, Acetone, Acetophenone, Methanal, 4nitrobenzaldehyde, cyclohexane carbaldehyde.

The value of $$x$$ in compound 'D' is _________.
The mass of NH$$_3$$ produced when 131.8 kg of cyclohexanecarbaldehyde undergoes Tollen's test is ________ kg. (Nearest Integer)
Molar Mass of
C = 12g/mol
N = 14g/mol
O = 16g/mol
Number of isomeric compounds with molecular formula $$\mathrm{C}_{9} \mathrm{H}_{10} \mathrm{O}$$ which (i) do not dissolve in $$\mathrm{NaOH}$$ (ii) do not dissolve in $$\mathrm{HCl}$$. (iii) do not give orange precipitate with 2,4-DNP (iv) on hydrogenation give identical compound with molecular formula $$\mathrm{C}_{9} \mathrm{H}_{12} \mathrm{O}$$ is ____________.
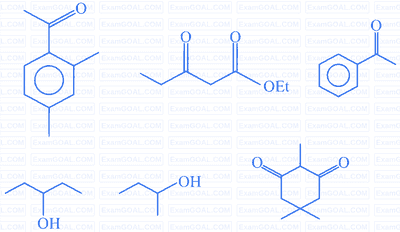
Number of compounds from the following which will not dissolve in cold $\mathrm{NaHCO}_{3}$ and $\mathrm{NaOH}$ solutions but will dissolve in hot $\mathrm{NaOH}$ solution is ________.

A trisubstituted compound '$$\mathrm{A}$$', $$\mathrm{C}_{10} \mathrm{H}_{12} \mathrm{O}_{2}$$ gives neutral $$\mathrm{FeCl}_{3}$$ test positive. Treatment of compound 'A' with $$\mathrm{NaOH}$$ and $$\mathrm{CH}_{3} \mathrm{Br}$$ gives $$\mathrm{C}_{11} \mathrm{H}_{14} \mathrm{O}_{2}$$, with hydroiodic acid gives methyl iodide and with hot conc. $$\mathrm{NaOH}$$ gives a compound $$\mathrm{B}, \mathrm{C}_{10} \mathrm{H}_{12} \mathrm{O}_{2}$$. Compound 'A' also decolorises alkaline $$\mathrm{KMnO}_{4}$$. The number of $$\pi$$ bond/s present in the compound '$$\mathrm{A}$$' is _____________.
Number of compounds giving (i) red colouration with ceric ammonium nitrate and also (ii) positive iodoform test from the following is ___________

The number of stereoisomers formed in a reaction of $$(±)\mathrm{Ph}(\mathrm{C}=\mathrm{O}) \mathrm{C}(\mathrm{OH})(\mathrm{CN}) \mathrm{Ph}$$ with $$\mathrm{HCN}$$ is ___________.
$$\left[\right.$$where $$\mathrm{Ph}$$ is $$-\mathrm{C}_{6} \mathrm{H}_{5}$$]
The spin only magnetic moment of the complex present in Fehling's reagent is __________ B.M. (Nearest integer).
In the given reaction

The number of chiral carbon/s in product A is ___________.
A hydrocarbon 'X' is found to have molar mass of 80. A 10.0 mg of compound 'X' on hydrogenation consumed 8.40 mL of H2 gas (measured at STP). Ozonolysis of compound 'X' yields only formaldehyde and dialdehyde. The total number of fragments/molecules produced from the ozonolysis of compound 'X' is _____________.
In the given reaction,

The number of $$\pi$$ electrons present in the product 'P' is _________.

Consider the above reaction where 6.1 g of Benzoic acid is used to get 7.8 g of m-bromo benzoic acid. The percentage yield of the product is __________
(Round off to the Nearest Integer).
[Given : Atomic masses : C : 12.0 u, H : 1.0 u, O : 16.0 u, Br : 80.0 u]
(A) Sulphanilic acid
(B) Picric acid
(C) Aspirin
(D) Ascorbic acid
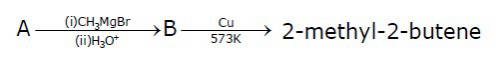
The mass percentage of carbon in A is ______.
MCQ (More than One Correct Answer)
A molecule ("P") on treatment with acid undergoes rearrangement and gives ("Q"). ("Q") on ozonolysis followed by reflux under alkaline condition gives (" $R$ "). The structure of (" $R$ ") is given below.

The structure of ("P") is
CrO3 - H2SO4 produced [B]. Which of the following strucutres are not possible for [A]?