Basics of Organic Chemistry · Chemistry · JEE Main
MCQ (Single Correct Answer)
Match the LIST-I with LIST-II
| LIST-I | LIST-II |
|---|---|
| A. Carbocation | I. Species that can supply a pair of electrons. |
| B. C-Free radical | II. Species that can receive a pair of electrons. |
| C. Nucleophile | III. sp2 hybridized carbon with empty p-orbital. |
| D. Electrophile | IV. sp2/sp3 hybridized carbon with one unpaired electron. |
Choose the correct answer from the options given below:
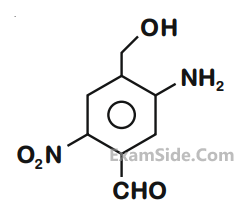
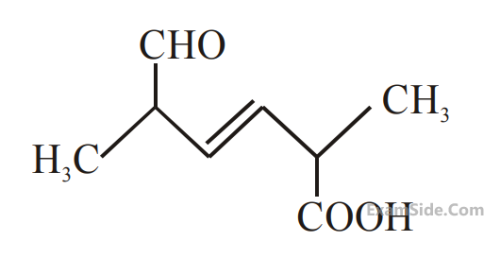
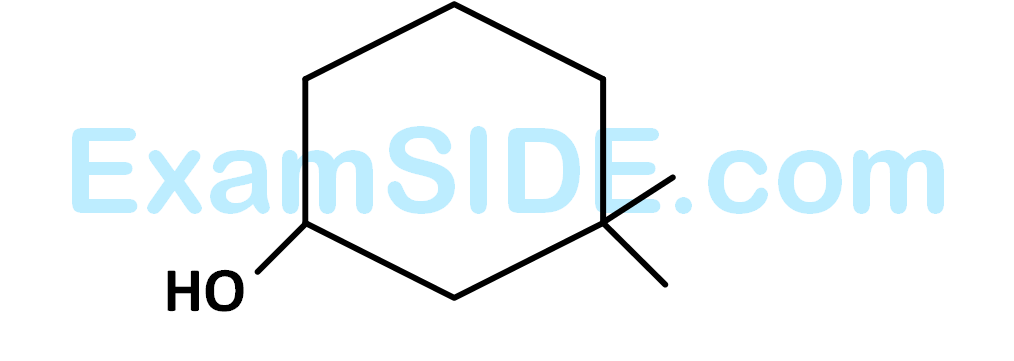
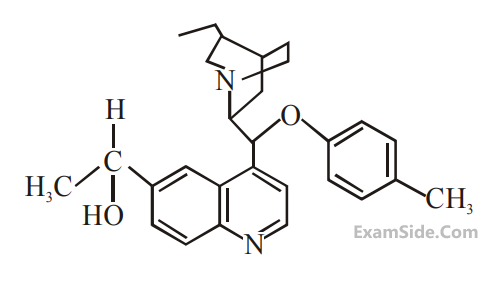
What is the correct IUPAC name of the compound?

The descending order of basicity of the following amines is :



(D) $ \mathrm{CH}_3 \mathrm{NH}_2$
(E) $\left(\mathrm{CH}_3\right)_2 \mathrm{NH}$
Choose the correct answer from the options given below :
The number of optically active products obtained from the complete ozonolysis of the given compound is :

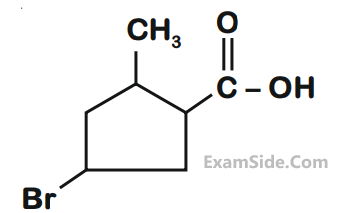
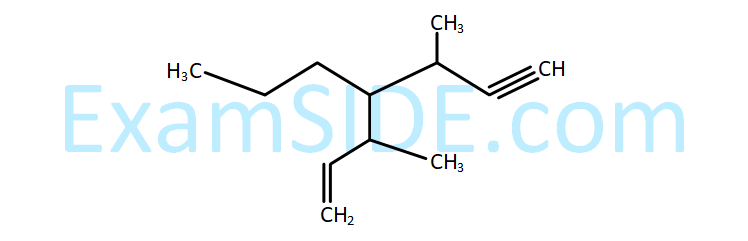
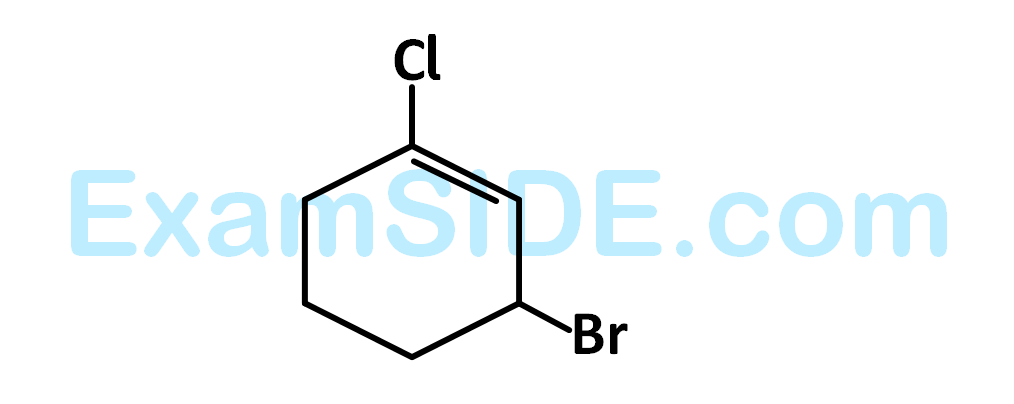
Which of the following is the correct IUPAC name of given organic compound (X)?

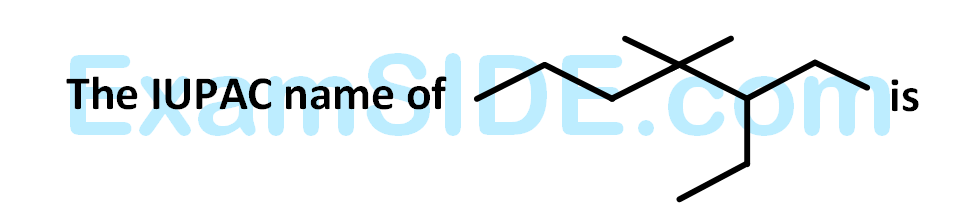
The IUPAC name of the following compound is:

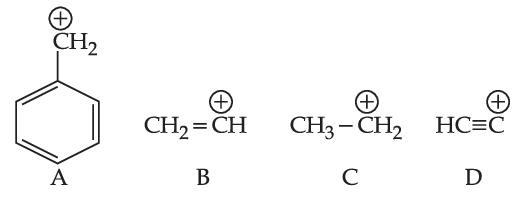
In which pairs, the first ion is more stable than the second?

The correct order of basicity for the following molecules is :

$$ \text { What is the correct IUPAC name of } $$
 ?
?
Given below are two statements :
Statement I : Hyperconjugation is not a permanent effect.
Statement II : In general, greater the number of alkyl groups attached to a positively charged Catom, greater is the hyperconjugation interaction and stabilization of the cation.
In the light of the above statements, choose the correct answer from the options given below
$$ \text {Identify the correct statements from the following. } $$

$$ \text {Choose the correct answer from the options given below: } $$
$$ \text { The least acidic compound, among the following is: } $$

Consider the following compound (X)

The most stable and least stable carbon radicals, respectively, produced by homolytic cleavage of corresponding $\mathrm{C}-\mathrm{H}$ bond are :
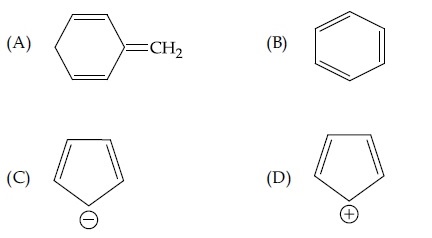
Designate whether each of the following compounds is aromatic or not aromatic.

Given below are two statements :
Statement (I): On nitration of m-xylene with $\mathrm{HNO}_3, \mathrm{H}_2 \mathrm{SO}_4$ followed by oxidation, 4-nitrobenzene-1,3-dicarboxylic acid is obtained as the major product.
Statement (II) : $-\mathrm{CH}_3$ group is o/p-directing while $-\mathrm{NO}_2$ group is m-directing group.
In the light of the above statements, choose the correct answer from the options given below :
Given below are two statements :
Statement (I): In partition chromatography, stationary phase is thin film of liquid present in the inert support.
Statement (II): In paper chromatography, the material of paper acts as a stationary phase.
In the light of the above statements, choose the correct answer from the options given below :
Match List - I with List - II.
| List - I (Structure) | List - II (IUPAC Name) |
|---|---|
(A)  |
(I) 4-Methylpent-1-ene |
| (B) (CH3)2C(C3H7)2 | (II) 3-Ethyl-5-methylheptane |
(C)  |
(III) 4,4-Dimethylheptane |
(D)  |
(IV) 2-Methyl-1,3-pentadiene |
Choose the correct answer from the options given below :
Total number of nucleophiles from the following is :

Given below are two statements:
Statement I: and
and  are isomeric compounds.
are isomeric compounds.
Statement II:  and
and  are functional group isomers.
are functional group isomers.
In the light of the above statements, choose the correct answer from the options given below:
The correct order of stability of following carbocations is :

In the given structure, number of sp and $\mathrm{sp}^2$ hybridized carbon atoms present respectively are :

Identify correct statement/s :
(A) $-\mathrm{OCH}_3$ and $-\mathrm{NHCOCH}_3$ are activating group.
(B) $\quad-\mathrm{CN}$ and -OH are meta directing group.
(C) -CN and $-\mathrm{SO}_3 \mathrm{H}$ are meta directing group.
(D) Activating groups act as ortho - and para directing groups.
(E) Halides are activating groups.
Choose the correct answer from the options given below :
Which one of the carbocations from the following is most stable?
The correct stability order of the following species/molecules is :

The most stable carbocation from the following is :
How many different stereoisomers are possible for the given molecule?

The incorrect statements regarding geometrical isomerism are :
(A) Propene shows geometrical isomerism.
(B) Trans isomer has identical atoms/groups on the opposite sides of the double bond.
(C) Cis-but-2-ene has higher dipole moment than trans-but-2-ene.
(D) 2-methylbut-2-ene shows two geometrical isomers.
(E) Trans-isomer has lower melting point than cis isomer.
Choose the correct answer from the options given below :
The IUPAC name of the following compound is :

The correct stability order of the following resonance structures of $$\mathrm{CH}_3-\mathrm{CH}=\mathrm{CH}-\mathrm{CHO}$$ is

Total number of stereo isomers possible for the given structure :

Relative stability of the contributing structures is :

Correct order of basic strength of Pyrrole  , Pyridine
, Pyridine  and Piperidine
and Piperidine  is :
is :
For the given compounds, the correct order of increasing $$\mathrm{pK}_{\mathrm{a}}$$ value :

Choose the correct answer from the options given below :
Methods used for purification of organic compounds are based on :
The correct sequence of acidic strength of the following aliphatic acids in their decreasing order is:
$$\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{COOH}, \mathrm{CH}_3 \mathrm{COOH}, \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{COOH}, \mathrm{HCOOH}$$
IUPAC name of following hydrocarbon(X) is :

Which of the following are aromatic?

Given below are two statements :

In the light of the above statements, choose the most appropriate answer from the options given below:

The correct arrangement for decreasing order of electrophilic substitution for above compounds is :
The incorrect statement regarding the geometrical isomers of 2-butene is :
Functional group present in sulphonic acids is :
Which of the following is metamer of the given compound (X) ?

The correct nomenclature for the following compound is :

Match List - I with List - II.
| LIST I (Pair of Compounds) |
LIST II (Isomerism) |
||
|---|---|---|---|
| A. | n-propanol and Isopropanol | I. | Metamerism |
| B. | Methoxypropane and ethoxyethane | II. | Chain Isomerism |
| C. | Propanone and propanal | III. | Position Isomerism |
| D. | Neopentane and Isopentane | IV. | Functional Isomerism |
Choose the correct answer from the options given below :
Given below are two statements : one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A) : Cis form of alkene is found to be more polar than the trans form.
Reason (R) : Dipole moment of trans isomer of 2-butene is zero.
In the light of the above statements, choose the correct answer from the options given below :
Correct order of stability of carbanion is :

What will be the decreasing order of basic strength of the following conjugate bases? $${ }^{-} \mathrm{OH}, \mathrm{R} \overline{\mathrm{O}}, \mathrm{CH}_3 \mathrm{CO} \overline{\mathrm{O}}, \mathrm{Cl}$$
Match List I with List II :
| LIST I Mechanism steps |
LIST II Effect |
||
|---|---|---|---|
| A. |  |
I. | $$-$$ E effect |
| B. |  |
II. | $$-$$ R effect |
| C. |  |
III. | $$+$$ E effect |
| D. |  |
IV. | $$+$$ R effect |
Choose the correct answer from the options given below :
Which among the following is incorrect statement?
(A) homolytic bond cleavage
(B) heterolytic bond cleavage
(C) free radical formation
(D) primary free radical
(E) secondary free radical
Choose the correct answer from the options given below :
Identify structure of 2,3-dibromo-1-phenylpentane.
The correct order of reactivity in electrophilic substitution reaction of the following compounds is :

A species having carbon with sextet of electrons and can act as electrophile is called
Given below are two statements:
Statement I: IUPAC name of $$\mathrm{HO}-\mathrm{CH}_2-\left(\mathrm{CH}_2\right)_3-\mathrm{CH}_2-\mathrm{COCH}_3$$ is 7-hydroxyheptan-2-one.
Statement II: 2-oxoheptan-7-ol is the correct IUPAC name for above compound. In the light of the above statements, choose the most appropriate answer from the options given below:
The correct stability order of carbocations is
Which among the following purification methods is based on the principle of "Solubility" in two different solvents?
IUPAC name of following compound is :

Which of the following molecule/species is most stable?
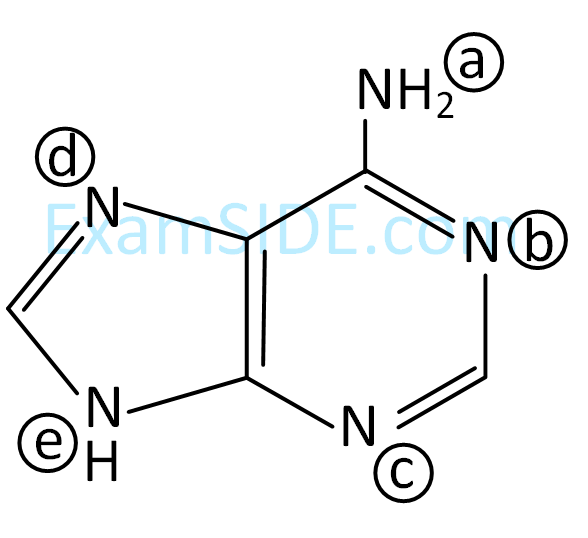
According to IUPAC system, the compound

is named as
The ascending acidity order of the following H atoms is

Which one of the following will show geometrical isomerism?
The interaction between $$\pi$$ bond and lone pair of electrons present on an adjacent atom is responsible for
The order of relative stability of the contributing structure is :

Choose the correct answer from the options given below :
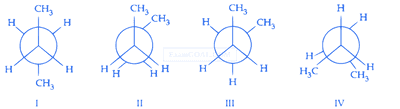
The incorrect statement regarding conformations of ethane is :
Bond line formula of HOCH(CN)$$_2$$ is :
Which of the following is strongest Bronsted base?
Which of the following has highly acidic hydrogen?
Cyclohexene

is ________ type of an organic compound.
IUPAC name of following compound (P) is:

Given below are two statements :
Statement I : Tropolone is an aromatic compound and has $$8 \pi$$ electrons.
Statement II : $$\pi$$ electrons of $$ > \mathrm{C}=\mathrm{O}$$ group in tropolone is involved in aromaticity.
In the light of the above statements, choose the correct answer from the options given below:
Among the following compounds, the one which shows highest dipole moment is
Thin layer chromatography of a mixture shows the following observation:

The correct order of elution in the silica gel column chromatography is
The decreasing order of hydride affinity for following carbocations is:

Choose the correct answer from the options given below:
Using column chromatography, mixture of two compounds 'A' and 'B' was separated. 'A' eluted first, this indicates 'B' has
The correct IUPAC nomenclature for the following compound is:

The descending order of acidity for the following carboxylic acid is-
A. $$\mathrm{CH}_{3} \mathrm{COOH}$$
B. $$\mathrm{F}_{3} \mathrm{C}-\mathrm{COOH}$$
C. $$\mathrm{ClCH}_{2}-\mathrm{COOH}$$
D. $$\mathrm{FCH}_{2}-\mathrm{COOH}$$
E. $$\mathrm{BrCH}_{2}-\mathrm{COOH}$$
Choose the correct answer from the options given below:
From the figure of column chromatography given below, identify incorrect statements.

A. Compound 'c' is more polar than 'a' and 'b'
B. Compound '$$\mathrm{a}$$' is least polar
C. Compound 'b' comes out of the column before 'c' and after 'a'
D. Compound 'a' spends more time in the column
Choose the correct answer from the options given below:
Resonance in carbonate ion $$\left(\mathrm{CO}_{3}{ }^{2-}\right)$$ is

Which of the following is true?
Match items of column I and II
| Column I (Mixture of compounds) | Column II (Separation Technique) | ||
|---|---|---|---|
| A. | $$\mathrm{H_2O/CH_2Cl_2}$$ | i. | Crystalization |
| B. |  |
ii. | Differential solvent extraction |
| C. | Kerosene / Naphthalene | iii. | Column chromatography |
| D. | $$\mathrm{C_6H_{12}O_6/NaCl}$$ | iv. | Fractional Distillation |
Correct match is
Match List I with List II:
| List I (Mixture) | List II (Separation Technique) | ||
|---|---|---|---|
| A. | $\mathrm{CHCl}_3+\mathrm{C}_6 \mathrm{H}_5 \mathrm{NH}_2$ | I. | Steam distillation |
| B. | $\mathrm{C}_6 \mathrm{H}_{14}+\mathrm{C}_5 \mathrm{H}_{12}$ | II. | Differential extraction |
| C. | $\mathrm{C}_6 \mathrm{H}_5 \mathrm{NH}_2+\mathrm{H}_2 \mathrm{O}$ | III. | Distillation |
| D. | $\text { Organic compound in } \mathrm{H}_2 \mathrm{O}$ | IV. | Fractional distillation |
Which of the following conformations will be the most stable?
The correct order in aqueous medium of basic strength in case of methyl substituted amines is :
Given below are two statements, one is labelled as Assertion A and the other is labelled as Reason R
Assertion A : Benzene is more stable than hypothetical cyclohexatriene
Reason R : The delocalised $$\pi$$ electron cloud is attracted more strongly by nuclei of carbon atoms.
In the light of the above statements, choose the correct answer from the options given below:
Which will undergo deprotonation most readily in the basic medium?

Given below are two statements.
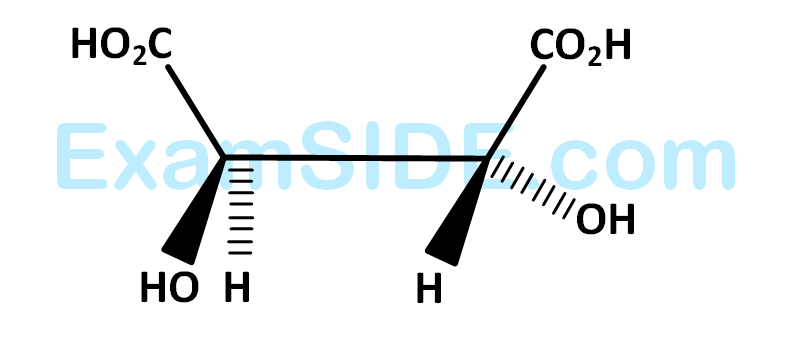
Statement I : The compound  is optically active.
is optically active.
Statement II :  is mirror image of above compound A.
is mirror image of above compound A.
In the light of the above statement, choose the most appropriate answer from the options given below.
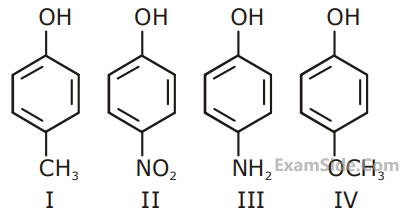
Arrange the following in increasing order of reactivity towards nitration
A. p-xylene
B. bromobenzene
C. mesitylene
D. nitrobenzene
E. benzene
Choose the correct answer from the options given below
Given below are two statements: One is labelled as Assertion A and the other is labelled as Reason R
Assertion A : Thin layer chromatography is an adsorption chromatography.
Reason R : A thin layer of silica gel is spread over a glass plate of suitable size in thin layer chromatography which acts as an adsorbent.
In the light of the above statements, choose the correct answer from the options given below
Match List - I with List - II.
| List - I | List - II | ||
|---|---|---|---|
| (A) |  |
(I) | Spiro compound |
| (B) |  |
(II) | Aromatic compound |
| (C) |  |
(III) | Non-planar Heterocyclic compound |
| (D) |  |
(IV) | Bicyclo compound |
Choose the correct answer from the options given below :
Among the following marked proton of which compound shows lowest $$\mathrm{pK}_{\mathrm{a}}$$ value?
Match List - I with List - II.
| List - I (Mixture) |
List - II (Purification Process) |
||
|---|---|---|---|
| (A) | Chloroform & Aniline | (I) | Steam distillation |
| (B) | Benzoic acid & Napthalene | (II) | Sublimation |
| (C) | Water & Aniline | (III) | Distillation |
| (D) | Napthalene & Sodium chloride | (IV) | Crystallisation |
Choose the correct answer from the options given below :
Given below are two statements: one is labelled as Assertion A and, the other is labelled as Reason R.
Assertion A: [6] Annulene, [8] Annulene and cis-[10] Annulene, are respectively aromatic, not-aromatic and aromatic.

Reason R: Planarity is one of the requirements of aromatic systems.
In the light of the above statements, choose the most appropriate answer from the options given below.
The correct decreasing order of priority of functional groups in naming an organic Question: compound as per IUPAC system of nomenclature is
Which technique among the following, is most appropriate in separation of a mixture of $$100 \,\mathrm{mg}$$ of $$p$$-nitrophenol and picric acid ?
Which of the following compounds is not aromatic?
Arrange the following in decreasing acidic strength.

Which of the following carbocations is most stable?
The correct IUPAC name of the following compound is :

Which one of the following techniques is not used to spot components of a mixture separated on thin layer chromatographic plate?
Which of the following structure are aromatic in nature?

Which of the following is most stable?
The correct order of nucleophilicity is
Given below are two statements : one is labelled as Assertion A and the other is labelled as Reason R.
Assertion A : A mixture contains benzoic acid and napthalene. The pure benzoic acid can be separated out by the use of benzene.
Reason R : Benzoic acid is soluble in hot water.
In the light of the above statements, choose the most appropriate answer from the options given below.
Phenol on reaction with dilute nitric acid, gives two products. Which method will be most efficient for large scale separation?
In the following structures, which on is having staggered conformation with maximum dihedral angle?
The IUPAC name of ethylidene chloride is :
Arrange the following carbocations in decreasing order of stability.

Which of the following is an example of conjugated diketone?

Assertion (A) : A simple distillation can be used to separate a mixture of propanol and propanone.
Reason (R) : Two liquids with a difference of more than 20$$^\circ$$ in their boiling points can be separated by simple distillations.
In the light of the above statements, choose the most appropriate answer from the options given below :
Statement I : Hyperconjugation is a permanent effect.
Statement II : Hyperconjugation in ethyl cation $$\left( {C{H_3} - \mathop C\limits^ + {H_2}} \right)$$ involves the overlapping of $${C_{s{p^2}}} - {H_{1s}}$$ bond with empty 2p orbital of other carbon.
Choose the correct option :
The correct order of stability of given carbocation is :

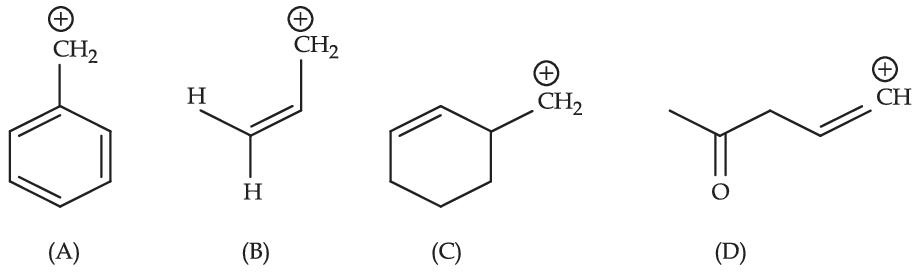
Among the given species the Resonance stabilised carbocations are :

Hybridisation of Carbon a, b, and c respectively are :
Statement I : Retardation factor (Rf) can be measured in meter/centimeter.
Statement II : Rf value of a compound remains constant in all solvents.
Choose the most appropriate answer from the options given below :
Choose the correct answer from the following options :
Statement I : A mixture of chloroform and aniline can be separated by simple distillation.
Statement II : When separating aniline from a mixture of aniline and water by steam distillation aniline boils below its boiling point.
In the light of the above statements, choose the most appropriate answer from the options given below :







(a) CH3CO2$$-$$ (b) H2O
(c) CH3SO3$$-$$ (d) $$\mathop O\limits^ - H$$




the favourable site/s for protonation is/are :
| Item-I (Mixture) | Item-II (Seperation method) | ||
|---|---|---|---|
| (A) | H2O : Sugar | (P) | Sublimation |
| (B) | H2O : Aniline | (Q) | Recrystallization |
| (C) | H2O : Toluene |
(R) | Steam distillation |
| (S) | Differential extraction | ||



| List - I | List - II | ||
|---|---|---|---|
| (A) | Coloured impurity | (P) | Steam distilation |
| (B) | Mixture of o-nitrophenol and p-nitrophenol |
(Q) | Fractional distilation |
| (C) | Crude Naphtha | (R) | Charcoal treatment |
| (d) | Mixture of glycerol and sugars |
(S) | Distillation under reduced pressure |



Geometrical isomerism is not possible at site (s) :



is :

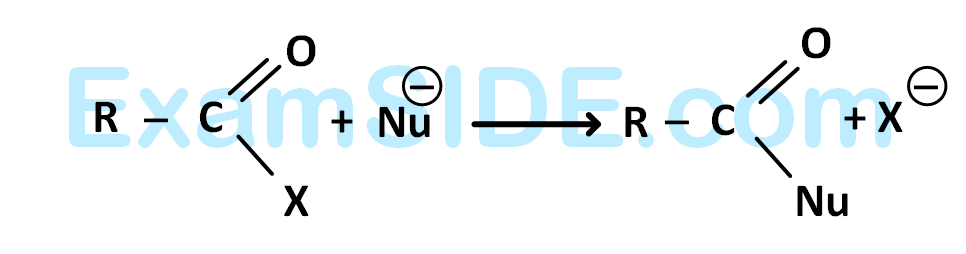
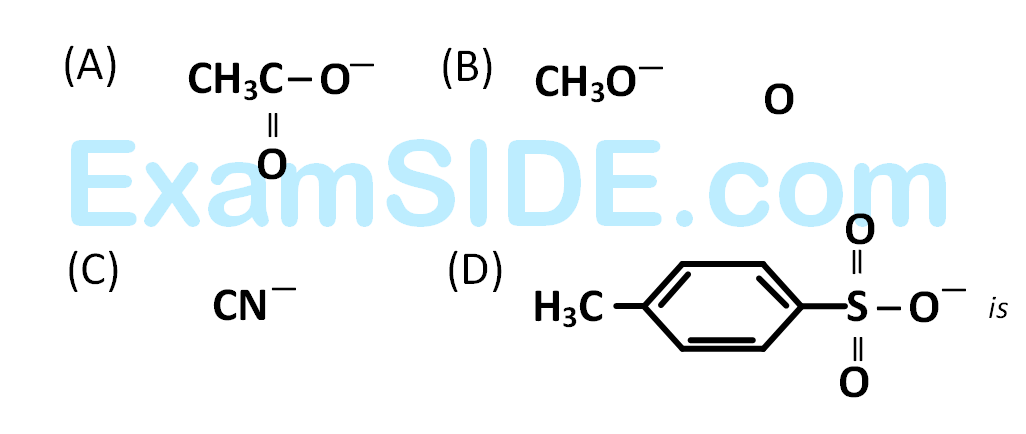
[Nu– = (A) PhO–, (B) AcO–, (C) HO–, (D) CH3O–]
is fastest when $$X$$ is
(a) PhCOOH
(b) o – NO2C6H4COOH
(c) p – NO2C6H4COOH
(d) m – NO2C6H4COOH
Which of the following order is correct?
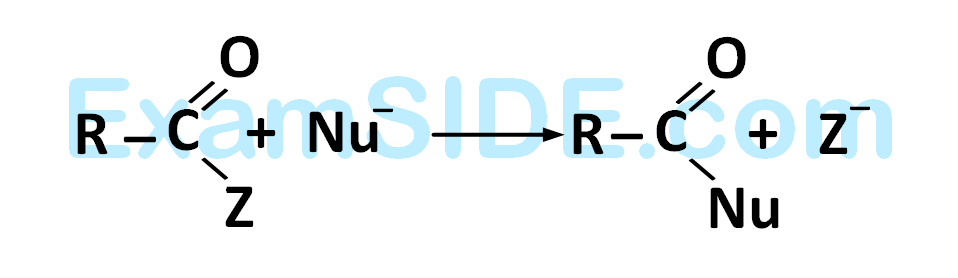
is fastest when $$Z$$ is
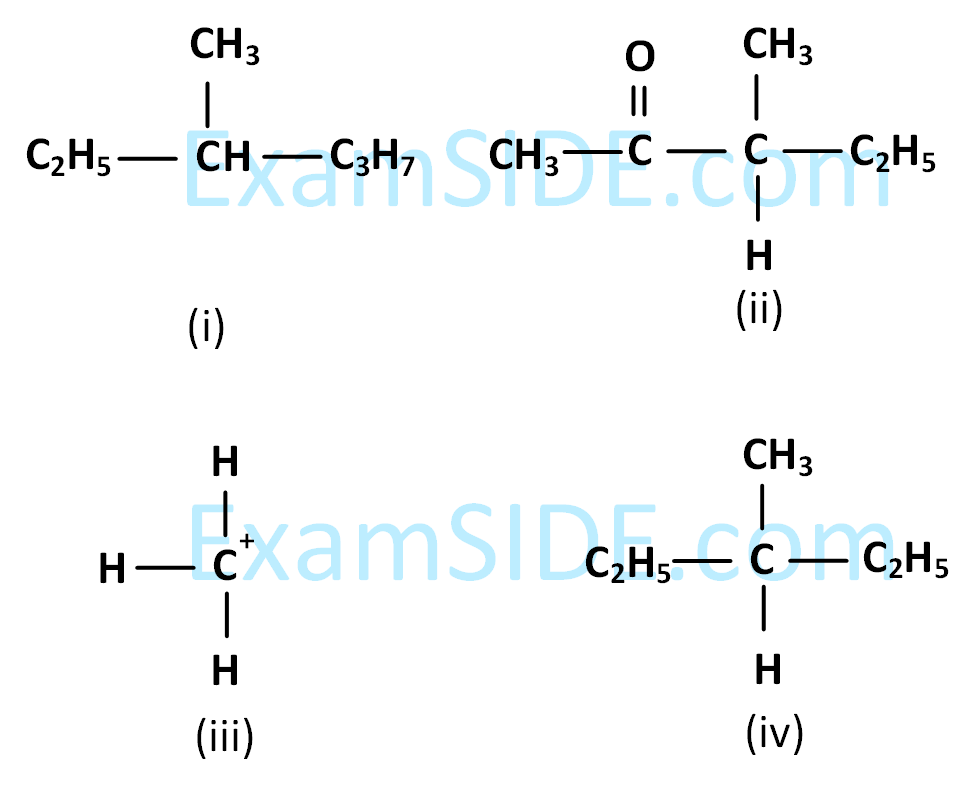
it is true that

are studied in terms of isomerism in :
Numerical
The total number of structural isomers possible for the substituted benzene derivatives with the molecular formula $\mathrm{C}_9 \mathrm{H}_{12}$ is________.
The hydrocarbon $(\mathrm{X})$ with molar mass $80 \mathrm{~g} \mathrm{~mol}^{-1}$ and $90 \%$ carbon has _______ degree of unsaturation.
The possible number of stereoisomers for 5-phenylpent-4-en-2-ol is ________.
In Carius method for estimation of halogens, 180 mg of an organic compound produced 143.5 mg of AgCl . The percentage composition of chlorine in the compound is _________ %. (Given : molar mass in $\mathrm{g} \mathrm{~mol}^{-1}$ of $\mathrm{Ag}: 108, \mathrm{Cl}: 35.5$ )
How many compounds among the following compounds show inductive, mesomeric as well as hyperconjugation effects?

Total number of aromatic compounds among the following compounds is ______.

The number of optically active compounds from the following is _________.

The number of optical isomers in following compound is : __________.

Number of carbocations from the following that are not stabilized by hyperconjugation is _______.

Using the given figure, the ratio of $$\mathrm{R}_f$$ values of sample $$\mathrm{A}$$ and sample $$\mathrm{C}$$ is $$x \times 10^{-2}$$. Value of $$x$$ is __________.

The total number of 'sigma' and 'pi' bonds in 2-oxohex-4-ynoic acid is ______.
The number of different chain isomers for C$$_7$$H$$_{16}$$ is __________.

Number of isomeric products formed by monochlorination of 2-methylbutane in presence of sunlight is ________.
Number of geometrical isomers possible for the given structure is/are _________.

Total number of compounds with Chiral carbon atoms from following is _________.

3-Methylhex-2-ene on reaction with $$\mathrm{HBr}$$ in presence of peroxide forms an addition product (A). The number of possible stereoisomers for '$$\mathrm{A}$$' is ________.
Among the given organic compounds, the total number of aromatic compounds is

Among the following, total number of meta directing functional groups is (Integer based)
$$-\mathrm{OCH}_3,-\mathrm{NO}_2,-\mathrm{CN},-\mathrm{CH}_3-\mathrm{NHCOCH}_3, -\mathrm{COR},-\mathrm{OH},-\mathrm{COOH},-\mathrm{Cl}$$
Three organic compounds A, B and $$\mathrm{C}$$ were allowed to run in thin layer chromatography using hexane and gave the following result (see figure). The $$\mathrm{R}_{\mathrm{f}}$$ value of the most polar compound is ____________ $$\times 10^{-2}$$

The total number of chiral compound/s from the following is ______________.

Following chromatogram was developed by adsorption of compound 'A' on a 6 cm TLC glass plate. Retardation factor of the compound 'A' is _________ $$\times 10^{-1}$$.

Optical activity of an enantiomeric mixture is $$+12.6^{\circ}$$ and the specific rotation of $$(+)$$ isomer is $$+30^{\circ}$$. The optical purity is __________$$\%$$.
Total number of isomers (including stereoisomers) obtained on monochlorination of methylcyclohexane is ___________.
The separation of two coloured substances was done by paper chromatography. The distances travelled by solvent front, substance A and substance B from the base line are 3.25 cm, 2.08 cm and 1.05 cm, respectively. The ratio of Rf values of A to B is _____________.
The total number of monobromo derivatives formed by the alkanes with molecular formula C5H12 is (excluding stereo isomers) __________.
Observe structures of the following compounds

The total number of structures/compounds which possess asymmetric carbon atoms is ______________.
Total number of possible stereoisomers of dimethyl cyclopentane is ____________.
Number of electrophilic centres in the given compound is _______________.

[Atomic mass : Silver = 108, Bromine = 80]

Fig : Paper chromatography for compounds A and B.
the calculated Rf value of A ________$$\times$$ 10-1.
MCQ (More than One Correct Answer)
Which of the following is not an example of benzenoid compound?