Electrochemistry · Chemistry · JEE Main
Numerical
Consider the following half cell reaction
$$ \text{Cr}_2\text{O}_7^{2-} \, (\text{aq}) + 6\text{e}^- + 14\text{H}^+ \, (\text{aq}) \rightarrow 2\text{Cr}^{3+} \, (\text{aq}) + 7\text{H}_2\text{O} \, (\ell) $$
The reaction was conducted with the ratio of $$\frac{[\text{Cr}^{3+}]^2}{[\text{Cr}_2\text{O}_7^{2-}]} = 10^{-6}$$. The pH value at which the EMF of the half cell will become zero is __________.
(nearest integer value)
[Given: standard half cell reduction potential $$E^{\circ}_{\text{Cr}_2\text{O}_7^{2-}, \text{H}^+/\text{Cr}^{3+}} = 1.33\, \text{V}$$, $$\frac{2.303RT}{F} = 0.059\, \text{V}$$.]
1 Faraday electricity was passed through $\mathrm{Cu}^{2+}(1.5 \mathrm{M}, 1 \mathrm{~L}) / \mathrm{Cu}$ and 0.1 Faraday was passed through $\mathrm{Ag}^{+}(0.2 \mathrm{M}, 1 \mathrm{~L}) / \mathrm{Ag}$ electrolytic cells. After this the two cells were connected as shown below to make an electrochemical cell. The emf of the cell thus formed at 298 K is __________ mV (nearest integer)

$$\begin{aligned} \text { Given : } \mathrm{E}^{\circ} \mathrm{Cu}^{2+} / \mathrm{Cu} & =0.34 \mathrm{~V} \\\\ \mathrm{E}^{\circ} \mathrm{Ag}^{+} / \mathrm{Ag} & =0.8 \mathrm{~V} \\\\ \frac{2 \cdot 303 \mathrm{RT}}{\mathrm{~F}} & =0.06 \mathrm{~V} \end{aligned}$$
$0.2 \%(\mathrm{w} / \mathrm{v})$ solution of NaOH is measured to have resistivity $870.0 \mathrm{~m} \Omega \mathrm{~m}$. The molar conductivity of the solution will be__________$\times 10^2 \mathrm{mS} \mathrm{dm}^2 \mathrm{~mol}^{-1}$. (Nearest integer)
Consider the following electrochemical cell at standard condition.
$$\mathrm{Au}(\mathrm{~s})\left|\mathrm{QH}_2, \mathrm{Q}\right| \mathrm{NH}_4 \mathrm{X}(0.01 \mathrm{M})| | \mathrm{Ag}^{+}(1 \mathrm{M}) \mid \mathrm{Ag}(\mathrm{~s}) \mathrm{E}_{\text {cell }}=+0.4 \mathrm{~V}$$
The couple $\mathrm{QH}_2 / \mathrm{Q}$ represents quinhydrone electrode, the half cell reaction is given below:

$$\left[\text { Given : } \mathrm{E}_{\mathrm{Ag}^{+} / \mathrm{Ag}}^0=+0.8 \mathrm{~V} \text { and } \frac{2.303 \mathrm{RT}}{\mathrm{~F}}=0.06 \mathrm{~V}\right]$$
The $\mathrm{pK}_{\mathrm{b}}$ value of the ammonium halide salt $\left(\mathrm{NH}_4 \mathrm{X}\right)$ used here is __________ . (nearest integer)
The current in Amperes used for the given electrolysis is ___________ . (Nearest integer).
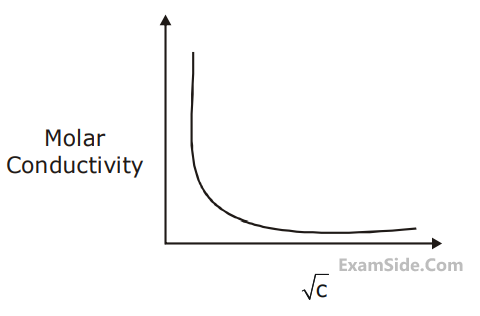
Given below is the plot of the molar conductivity vs $\sqrt{\text { concentration }}$ for KCl in aqueous solution.

If, for the higher concentration of KCl solution, the resistance of the conductivity cell is $100 \Omega$, then the resistance of the same cell with the dilute solution is ' x ' $\Omega$
The value of $x$ is _________ (Nearest integer)
The standard reduction potentials at $$298 \mathrm{~K}$$ for the following half cells are given below :
$$\mathrm{Cr}_2 \mathrm{O}_7^{2-}+14 \mathrm{H}^{+}+6 \mathrm{e}^{-} \rightarrow 2 \mathrm{Cr}^{3+}+7 \mathrm{H}_2 \mathrm{O}, \quad \mathrm{E}^{\circ}=1.33 \mathrm{~V}$$
$$\begin{array}{ll} \mathrm{Fe}^{3+}(\mathrm{aq})+3 \mathrm{e}^{-} \rightarrow \mathrm{Fe} & \mathrm{E}^{\circ}=-0.04 \mathrm{~V} \\ \mathrm{Ni}^{2+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{Ni} & \mathrm{E}^{\circ}=-0.25 \mathrm{~V} \\ \mathrm{Ag}^{+}(\mathrm{aq})+\mathrm{e}^{-} \rightarrow \mathrm{Ag} & \mathrm{E}^{\circ}=0.80 \mathrm{~V} \\ \mathrm{Au}^{3+}(\mathrm{aq})+3 \mathrm{e}^{-} \rightarrow \mathrm{Au} & \mathrm{E}^{\circ}=1.40 \mathrm{~V} \end{array}$$
Consider the given electrochemical reactions,
The number of metal(s) which will be oxidized be $$\mathrm{Cr}_2 \mathrm{O}_7^{2-}$$, in aqueous solution is _________.
$$ \mathrm{MnO}_4^{-}+\mathrm{H}^{+}+\mathrm{H}_2 \mathrm{C}_2 \mathrm{O}_4 \rightleftharpoons \mathrm{Mn}^{2+}+\mathrm{H}_2 \mathrm{O}+\mathrm{CO}_2 $$
The standard reduction potentials are given as below $\left(\mathrm{E}_{\text {red }}^0\right)$ :
$$ \begin{aligned} & \mathrm{E}_{\mathrm{MnO}_4^{-} / \mathrm{Mn}^{2+}}^{\circ}=+1.51 \mathrm{~V} \\\\ & \mathrm{E}_{\mathrm{CO}_2 / \mathrm{H}_2 \mathrm{C}_2 \mathrm{O}_4}^{\circ}=-0.49 \mathrm{~V} \end{aligned} $$
If the equilibrium constant of the above reaction is given as $\mathrm{K}_{\mathrm{eq}}=10^x$, then the value of $x=$ __________ (nearest integer)
$$ \begin{aligned} & 2 \mathrm{H}_{(\mathrm{aq})}^{+}+2 \mathrm{e}^{-} \longrightarrow \mathrm{H}_2(\mathrm{~g}) \\\\ & {\left[\mathrm{H}^{+}\right]=1 \mathrm{M}, \mathrm{P}_{\mathrm{H}_2}=2 \mathrm{~atm}} \end{aligned} $$
(Given : $2.303 \mathrm{RT} / \mathrm{F}=0.06 \mathrm{~V}, \log 2=0.3$ )
$1.0 \times 10^{-16}, 1.2 \times 10,3.91,1.5 \times 10^{-2}, 1 \times 10^{-7}, 1.0 \times 10^3$.
The number of conductors among the materials is _____________.
Number of alkanes obtained on electrolysis of a mixture of $$\mathrm{CH}_3 \mathrm{COONa}$$ and $$\mathrm{C}_2 \mathrm{H}_5 \mathrm{COONa}$$ is ________.
One Faraday of electricity liberates $$x \times 10^{-1}$$ gram atom of copper from copper sulphate. $$x$$ is ________.
A constant current was passed through a solution of $$\mathrm{AuCl}_4^{-}$$ ion between gold electrodes. After a period of 10.0 minutes, the increase in mass of cathode was $$1.314 \mathrm{~g}$$. The total charge passed through the solution is _______ $$\times 10^{-2} \mathrm{~F}$$.
(Given atomic mass of $$\mathrm{Au}=197$$)
The mass of zinc produced by the electrolysis of zine sulphate solution with a steady current of $$0.015 \mathrm{~A}$$ for 15 minutes is _________ $$\times 10^{-4} \mathrm{~g}$$.
(Atomic mass of zinc $$=65.4 \mathrm{~amu}$$)
The hydrogen electrode is dipped in a solution of $$\mathrm{pH}=3$$ at $$25^{\circ} \mathrm{C}$$. The potential of the electrode will be _________ $$\times 10^{-2} \mathrm{~V}$$.
$$\left(\frac{2.303 \mathrm{RT}}{\mathrm{F}}=0.059 \mathrm{~V}\right)$$
The mass of silver (Molar mass of $$\mathrm{Ag}: 108 \mathrm{~gmol}^{-1}$$ ) displaced by a quantity of electricity which displaces $$5600 \mathrm{~mL}$$ of $$\mathrm{O}_2$$ at S.T.P. will be ______ g.
(A) Conductivity always decreases with decrease in concentration for both strong and weak electrolytes.
(B) The number of ions per unit volume that carry current in a solution increases on dilution.
(C) Molar conductivity increases with decrease in concentration
(D) The variation in molar conductivity is different for strong and weak electrolytes
(E) For weak electrolytes, the change in molar conductivity with dilution is due to decrease in degree of dissociation.
At $$298 \mathrm{~K}$$, the standard reduction potential for $$\mathrm{Cu}^{2+} / \mathrm{Cu}$$ electrode is $$0.34 \mathrm{~V}$$.
Given : $$\mathrm{K}_{\mathrm{sp}} \mathrm{Cu}(\mathrm{OH})_{2}=1 \times 10^{-20}$$
Take $$\frac{2.303 \mathrm{RT}}{\mathrm{F}}=0.059 \mathrm{~V}$$
The reduction potential at $$\mathrm{pH}=14$$ for the above couple is $$(-) x \times 10^{-2} \mathrm{~V}$$. The value of $$x$$ is ___________
A metal surface of $$100 \mathrm{~cm}^{2}$$ area has to be coated with nickel layer of thickness $$0.001 \mathrm{~mm}$$. A current of $$2 \mathrm{~A}$$ was passed through a solution of $$\mathrm{Ni}\left(\mathrm{NO}_{3}\right)_{2}$$ for '$$\mathrm{x}$$' seconds to coat the desired layer. The value of $$\mathrm{x}$$ is __________. (Nearest integer) ( $$\rho_{\mathrm{Ni}}$$ (density of Nickel) is $$10 \mathrm{~g} \mathrm{~mL}$$, Molar mass of Nickel is $$60 \mathrm{~g} \mathrm{~mol}^{-1}$$ $$\left.\mathrm{F}=96500 ~\mathrm{C} ~\mathrm{mol}^{-1}\right)$$
The number of correct statements from the following is __________
A. $$\mathrm{E_{\text {cell }}}$$ is an intensive parameter
B. A negative $$\mathrm{E}^{\ominus}$$ means that the redox couple is a stronger reducing agent than the $$\mathrm{H}^{+} / \mathrm{H}_{2}$$ couple.
C. The amount of electricity required for oxidation or reduction depends on the stoichiometry of the electrode reaction.
D. The amount of chemical reaction which occurs at any electrode during electrolysis by a current is proportional to the quantity of electricity passed through the electrolyte.
In an electrochemical reaction of lead, at standard temperature, if $$\mathrm{E}^{0}\left(\mathrm{~Pb}^{2+} / \mathrm{Pb}\right)=\mathrm{m}$$ Volt and $$\mathrm{E}^{0}\left(\mathrm{~Pb}^{4+} / \mathrm{Pb}\right)=\mathrm{n}$$ Volt, then the value of $$\mathrm{E}^{0}\left(\mathrm{~Pb}^{2+} / \mathrm{Pb}^{4+}\right)$$ is given by $$\mathrm{m-x n}$$. The value of $$\mathrm{x}$$ is ___________. (Nearest integer)
The specific conductance of $$0.0025 ~\mathrm{M}$$ acetic acid is $$5 \times 10^{-5} \mathrm{~S} \mathrm{~cm}^{-1}$$ at a certain temperature. The dissociation constant of acetic acid is __________ $$\times ~10^{-7}$$ (Nearest integer)
Consider limiting molar conductivity of $$\mathrm{CH}_{3} \mathrm{COOH}$$ as $$400 \mathrm{~S} \mathrm{~cm}^{2} \mathrm{~mol}^{-1}$$
$$\mathrm{FeO_4^{2 - }\buildrel { + 2.2V} \over \longrightarrow F{e^{3 + }}\buildrel { + 0.70V} \over \longrightarrow F{e^{2 + }}\buildrel { - 0.45V} \over \longrightarrow F{e^0}}$$
$$E_{FeO_4^{2 - }/F{e^{2 + }}}^\theta $$ is $$x \times {10^{ - 3}}$$ V. The value of $$x$$ is _________
The number of incorrect statements from the following is ___________.
A. The electrical work that a reaction can perform at constant pressure and temperature is equal to the reaction Gibbs energy.
B. $$\mathrm{E_{cell}^{\circ}}$$ cell is dependent on the pressure.
C. $$\frac{d E^{\theta} \text { cell }}{\mathrm{dT}}=\frac{\Delta_{\mathrm{r}} \mathrm{S}^{\theta}}{\mathrm{nF}}$$
D. A cell is operating reversibly if the cell potential is exactly balanced by an opposing source of potential difference.
The standard reduction potentials at $$298 \mathrm{~K}$$ for the following half cells are given below:
$$\mathrm{NO}_{3}^{-}+4 \mathrm{H}^{+}+3 \mathrm{e}^{-} \rightarrow \mathrm{NO}(\mathrm{g})+2 \mathrm{H}_{2} \mathrm{O} \quad \mathrm{E}^{\theta}=0.97 \mathrm{~V}$$
$$\mathrm{V}^{2+}(\mathrm{aq})+2 \mathrm{e}^{-} \rightarrow \mathrm{V} \quad\quad\quad \mathrm{E}^{\theta}=-1.19 \mathrm{~V}$$
$$\mathrm{Fe}^{3+}(\mathrm{aq})+3 \mathrm{e}^{-} \rightarrow \mathrm{Fe} \quad\quad\quad \mathrm{E}^{\theta}=-0.04 \mathrm{~V}$$
$$\mathrm{Ag}^{+}(\mathrm{aq})+\mathrm{e}^{-} \rightarrow \mathrm{Ag}(\mathrm{s}) \quad\quad\quad \mathrm{E}^{\theta}=0.80 \mathrm{~V}$$
$$\mathrm{Au}^{3+}(\mathrm{aq})+3 \mathrm{e}^{-} \rightarrow \mathrm{Au}(\mathrm{s}) \quad\quad\quad \mathrm{E}^{\theta}=1.40 \mathrm{~V}$$
The number of metal(s) which will be oxidized by $$\mathrm{NO}_{3}^{-}$$ in aqueous solution is __________.
$$1 \times 10^{-5} ~\mathrm{M} ~\mathrm{AgNO}_{3}$$ is added to $$1 \mathrm{~L}$$ of saturated solution of $$\mathrm{AgBr}$$. The conductivity of this solution at $$298 \mathrm{~K}$$ is _____________ $$\times 10^{-8} \mathrm{~S} \mathrm{~m}^{-1}$$.
[Given : $$\mathrm{K}_{\mathrm{SP}}(\mathrm{AgBr})=4.9 \times 10^{-13}$$ at $$298 \mathrm{~K}$$
$$ \begin{aligned} & \lambda_{\mathrm{Ag}^{+}}^{0}=6 \times 10^{-3} \mathrm{~S} \mathrm{~m}^{2} \mathrm{~mol}^{-1} \\ & \lambda_{\mathrm{Br}^{-}}^{0}=8 \times 10^{-3} \mathrm{~S} \mathrm{~m}^{2} \mathrm{~mol}^{-1} \\ & \left.\lambda_{\mathrm{NO}_{3}^{-}}^{0}=7 \times 10^{-3} \mathrm{~S} \mathrm{~m}^{2} \mathrm{~mol}^{-1}\right] \end{aligned} $$
At what pH, given half cell $$\mathrm{MnO_{4}^{-}(0.1~M)~|~Mn^{2+}(0.001~M)}$$ will have electrode potential of 1.282 V? ___________ (Nearest Integer)
Given $$\mathrm{E_{MnO_4^ - |M{n^{2 + }}}^o}=1.54~\mathrm{V},\frac{2.303\mathrm{RT}}{\mathrm{F}}=0.059\mathrm{V}$$
Its molar conductivity is _________ $\times 10^{4}~ \Omega^{-1} \mathrm{~cm}^{2} \mathrm{~mol}^{-1}$. (Nearest integer)
The logarithm of equilibrium constant for the reaction $$\mathrm{Pd}^{2+}+4 \mathrm{Cl}^{-} \rightleftharpoons \mathrm{PdCl}_{4}^{2-}$$ is ___________ (Nearest integer)
Given : $$\frac{2.303 R \mathrm{~T}}{\mathrm{~F}}=0.06 \mathrm{~V}$$
$$ \mathrm{Pd}_{(\mathrm{aq})}^{2+}+2 \mathrm{e}^{-} \rightleftharpoons \mathrm{Pd}(\mathrm{s}) \quad \mathrm{E}^{\ominus}=0.83 \mathrm{~V} $$
$$ \begin{aligned} & \mathrm{PdCl}_{4}^{2-}(\mathrm{aq})+2 \mathrm{e}^{-} \rightleftharpoons \mathrm{Pd}(\mathrm{s})+4 \mathrm{Cl}^{-}(\mathrm{aq}) \mathrm{E}^{\ominus}=0.65 \mathrm{~V} \end{aligned} $$
$\mathrm{X}\left|\mathrm{X}^{2+}(0.001 \mathrm{M}) \| \mathrm{Y}^{2+}(0.01 \mathrm{M})\right| \mathrm{Y}$ is _______ $\times 10^{-2} \mathrm{~V}$ (Nearest integer)
Given: $\mathrm{E}^{0} _ {\mathrm{X}^{2+} \mid \mathrm{X}}=-2.36 \mathrm{~V}$
$\mathrm{E}_{\mathrm{Y}^{2+} \mid \mathrm{Y}}^{0}=+0.36 \mathrm{~V}$
$\frac{2.303 \mathrm{RT}}{\mathrm{F}}=0.06 \mathrm{~V}$
Consider the cell
$$\mathrm{Pt}_{(\mathrm{s})}\left|\mathrm{H}_{2}(\mathrm{~g}, 1 \mathrm{~atm})\right| \mathrm{H}^{+}(\mathrm{aq}, 1 \mathrm{M})|| \mathrm{Fe}^{3+}(\mathrm{aq}), \mathrm{Fe}^{2+}(\mathrm{aq}) \mid \operatorname{Pt}(\mathrm{s})$$
When the potential of the cell is $$0.712 \mathrm{~V}$$ at $$298 \mathrm{~K}$$, the ratio $$\left[\mathrm{Fe}^{2+}\right] /\left[\mathrm{Fe}^{3+}\right]$$ is _____________. (Nearest integer)
Given : $$\mathrm{Fe}^{3+}+\mathrm{e}^{-}=\mathrm{Fe}^{2+}, \mathrm{E}^{\theta} \mathrm{Fe}^{3+}, \mathrm{Fe}^{2+} \mid \mathrm{Pt}=0.771$$
$$ \frac{2.303 \mathrm{RT}}{\mathrm{F}}=0.06 \mathrm{~V} $$
The equilibrium constant for the reaction
$$\mathrm{Zn(s)+Sn^{2+}(aq)}$$ $$\rightleftharpoons$$ $$\mathrm{Zn^{2+}(aq)+Sn(s)}$$ is $$1\times10^{20}$$ at 298 K. The magnitude of standard electrode potential of $$\mathrm{Sn/Sn^{2+}}$$ if $$\mathrm{E_{Z{n^{2 + }}/Zn}^\Theta = - 0.76~V}$$ is __________ $$\times 10^{-2}$$ V. (Nearest integer)
Given : $$\mathrm{\frac{2.303RT}{F}=0.059~V}$$
Following figure shows dependence of molar conductance of two electrolytes on concentration. $$\Lambda \mathop m\limits^o $$ is the limiting molar conductivity.

The number of $$\mathrm{\underline {incorrect} }$$ statement(s) from the following is ___________
(A) $$\Lambda \mathop m\limits^o $$ for electrolyte A is obtained by extrapolation
(B) For electrolyte B, $$\Lambda \mathop m\limits $$ vs $$\sqrt c$$ graph is a straight line with intercept equal to $$\Lambda \mathop m\limits^o $$
(C) At infinite dilution, the value of degree of dissociation approaches zero for electrolyte B.
(D) $$\Lambda \mathop m\limits^o $$ for any electrolyte A and B can be calculated using $$\lambda^\circ$$ for individual ions
$$Pt(s)|{H_2}(g)(1\,bar)|{H^ + }(aq)(1\,M)||{M^{3 + }}(aq),{M^ + }(aq)|Pt(s)$$
The $$\mathrm{E_{cell}}$$ for the given cell is 0.1115 V at 298 K when $${{\left[ {{M^ + }(aq)} \right]} \over {\left[ {{M^{3 + }}(aq)} \right]}} = {10^a}$$
The value of $$a$$ is ____________
Given : $$\mathrm{E_{{M^{3 + }}/{M^ + }}^\theta = 0.2}$$ V
$${{2.303RT} \over F} = 0.059V$$
Consider the cell
$$\mathrm{Pt(s)|{H_2}(g)\,(1\,atm)|{H^ + }\,(aq,[{H^ + }] = 1)||F{e^{3 + }}(aq),F{e^{2 + }}(aq)|Pt(s)}$$
Given $$\mathrm{E_{F{e^{3 + }}/F{e^{2 + }}}^o = 0.771\,V}$$ and $$\mathrm{E_{{H^ + }/1/2\,{H_2}}^o = 0\,V,\,T = 298\,K}$$
If the potential of the cell is 0.712 V, the ratio of concentration of Fe$$^{2+}$$ to Fe$$^{3+}$$ is _____________ (Nearest integer)
At 298 K, a 1 litre solution containing 10 mmol of $$\mathrm{C{r_2}O_7^{2 - }}$$ and 100 mmol of $$\mathrm{Cr^{3+}}$$ shows a pH of 3.0.
Given : $$\mathrm{C{r_2}O_7^{2 - } \to C{r^{3 + }}\,;\,E^\circ = 1.330}$$V
and $$\mathrm{{{2.303\,RT} \over F} = 0.059}$$ V
The potential for the half cell reaction is $$x\times10^{-3}$$ V. The value of $$x$$ is __________
For a cell, $$\mathrm{Cu}(\mathrm{s})\left|\mathrm{Cu}^{2+}(0.001 \,\mathrm{M}) \| \mathrm{Ag}^{+}(0.01 \,\mathrm{M})\right| \mathrm{Ag}(\mathrm{s})$$
the cell potential is found to be $$0.43 \mathrm{~V}$$ at $$298 \mathrm{~K}$$. The magnitude of standard electrode potential for $$\mathrm{Cu}^{2+} / \mathrm{Cu}$$ is _________ $$\times 10^{-2} \mathrm{~V}$$.
[Given : $$E_{A{g^ + }/Ag}^\Theta $$ = 0.80 V and $${{2.303RT} \over F}$$ = 0.06 V]
Resistance of a conductivity cell (cell constant $$129 \mathrm{~m}^{-1}$$) filled with $$74.5 \,\mathrm{ppm}$$ solution of $$\mathrm{KCl}$$ is $$100 \,\Omega$$ (labelled as solution 1). When the same cell is filled with $$\mathrm{KCl}$$ solution of $$149 \,\mathrm{ppm}$$, the resistance is $$50 \,\Omega$$ (labelled as solution 2). The ratio of molar conductivity of solution 1 and solution 2 is i.e. $$\frac{\wedge_{1}}{\wedge_{2}}=x \times 10^{-3}$$. The value of $$x$$ is __________. (Nearest integer)
Given, molar mass of $$\mathrm{KCl}$$ is $$74.5 \mathrm{~g} \mathrm{~mol}^{-1}$$.
The amount of charge in $$\mathrm{F}$$ (Faraday) required to obtain one mole of iron from $$\mathrm{Fe}_{3} \mathrm{O}_{4}$$ is ___________. (Nearest Integer)
The spin-only magnetic moment value of M3+ ion (in gaseous state) from the pairs Cr3+ / Cr2+, Mn3+ / Mn2+, Fe3+ / Fe2+ and Co3+ / Co2+ that has negative standard electrode potential, is ____________ B.M. [Nearest integer]
The cell potential for $$\mathrm{Zn}\left|\mathrm{Zn}^{2+}(\mathrm{aq})\right|\left|\mathrm{Sn}^{x+}\right| \mathrm{Sn}$$ is $$0.801 \mathrm{~V}$$ at $$298 \mathrm{~K}$$. The reaction quotient for the above reaction is $$10^{-2}$$. The number of electrons involved in the given electrochemical cell reaction is ____________.
$$\left(\right.$$ Given $$: \mathrm{E}_{\mathrm{Zn}^{2+} \mid \mathrm{Zn}}^{\mathrm{o}}=-0.763 \mathrm{~V}, \mathrm{E}_{\mathrm{Sn}^{x+} \mid \mathrm{Sn}}^{\mathrm{o}}=+0.008 \mathrm{~V}$$ and $$\left.\frac{2.303 \mathrm{RT}}{\mathrm{F}}=0.06 \mathrm{~V}\right)$$
The cell potential for the given cell at 298 K
Pt| H2 (g, 1 bar) | H+ (aq) || Cu2+ (aq) | Cu(s)
is 0.31 V. The pH of the acidic solution is found to be 3, whereas the concentration of Cu2+ is 10$$-$$x M. The value of x is ___________.
(Given : $$E_{C{u^{2 + }}/Cu}^\Theta $$ = 0.34 V and $${{2.303\,RT} \over F}$$ = 0.06 V)
A dilute solution of sulphuric acid is electrolysed using a current of 0.10 A for 2 hours to produce hydrogen and oxygen gas. The total volume of gases produced a STP is _____________ cm3. (Nearest integer)
[Given : Faraday constant F = 96500 C mol$$-$$1 at STP, molar volume of an ideal gas is 22.7 L mol$$-$$1]
For the given reactions
Sn2+ + 2e$$-$$ $$\to$$ Sn
Sn4+ + 4e$$-$$ $$\to$$ Sn
the electrode potentials are ; $$E_{S{n^{2 + }}/Sn}^o = - 0.140$$ V and $$E_{S{n^{4 + }}/Sn}^o = + 0.010$$ V. The magnitude of standard electrode potential for $$S{n^{4 + }}/S{n^{2 + }}$$ i.e. $$E_{S{n^{4 + }}/S{n^{2 + }}}^o$$ is _____________ $$\times$$ 10$$-$$2 V. (Nearest integer)
The quantity of electricity in Faraday needed to reduce 1 mol of Cr2O$$_7^{2 - }$$ to Cr3+ is ____________.
For the reaction taking place in the cell :
Pt (s)| H2 (g)|H+(aq) || Ag+(aq) |Ag (s)
E$$_{cell}^o$$ = + 0.5332 V.
The value of $$\Delta$$fG$$^\circ$$ is ______________ kJ mol$$-$$1. (in nearest integer)
The limiting molar conductivities of NaI, NaNO3 and AgNO3 are 12.7, 12.0 and 13.3 mS m2 mol$$-$$1, respectively (all at 25$$^\circ$$C). The limiting molar conductivity of AgI at this temperature is ____________ mS m2 mol$$-$$1.
Cu(s) + Sn2+ (0.001M) $$\to$$ Cu2+ (0.01M) + Sn(s)
The Gibbs free energy change for the above reaction at 298 K is x $$\times$$ 10$$-$$1 kJ mol$$-$$1. The value of x is __________. [nearest integer]
[Given : $$E_{C{u^{2 + }}/Cu}^\Theta = 0.34\,V$$ ; $$E_{S{n^{2 + }}/Sn}^\Theta = - 0.14\,V$$ ; F = 96500 C mol$$-$$1]
A solution of Fe2(SO4)3 is electrolyzed for 'x' min with a current of 1.5 A to deposit 0.3482 g of Fe. The value of x is ___________. [nearest integer]
Given : 1 F = 96500 C mol$$-$$1
Atomic mass of Fe = 56 g mol$$-$$1
In a cell, the following reactions take place
$$\matrix{ {F{e^{2 + }} \to F{e^{3 + }} + {e^ - }} & {E_{F{e^{3 + }}/F{e^{2 + }}}^o = 0.77\,V} \cr {2{I^ - } \to {I_2} + 2{e^ - }} & {E_{{I_2}/{I^ - }}^o = 0.54\,V} \cr } $$
The standard electrode potential for the spontaneous reaction in the cell is x $$\times$$ 10$$-$$2 V 298 K. The value of x is ____________. (Nearest Integer)
The resistance of a conductivity cell containing 0.01 M KCl solution at 298 K is 1750 $$\Omega$$. If the conductivity of 0.01 M KCl solution at 298 K is 0.152 $$\times$$ 10$$-$$3 S cm$$-$$1, then the cell constant of the conductivity cell is ____________ $$\times$$ 10$$-$$3 cm$$-$$1.
The cell potential for the following cell
Pt |H2(g)|H+ (aq)|| Cu2+ (0.01 M)|Cu(s)
is 0.576 V at 298 K. The pH of the solution is __________. (Nearest integer)
(Given : $$E_{C{u^{2 + }}/Cu}^o = 0.34$$ V and $${{2.303\,RT} \over F} = 0.06$$ V)
$$C{d_{(s)}} + H{g_2}S{O_{4(s)}} + {9 \over 5}{H_2}{O_{(l)}}$$ $$\rightleftharpoons$$ $$CdS{O_4}.{9 \over 5}{H_2}{O_{(s)}} + 2H{g_{(l)}}$$
The value of $$E_{cell}^0$$ is 4.315 V at 25$$^\circ$$C. If $$\Delta$$H$$^\circ$$ = $$-$$825.2 kJ mol$$-$$1, the standard entropy change $$\Delta$$S$$^\circ$$ in J K$$-$$1 is ___________. (Nearest integer) [Given : Faraday constant = 96487 C mol$$-$$1]
Zn(s) + Cu2+ (0.02 M) $$\to$$ Zn2+ (0.04 M) + Cu(s),
Ecell = ______________ $$\times$$ 10$$-$$2 V. (Nearest integer)
[Use : $$E_{Cu/C{u^{2 + }}}^0$$ = $$-$$ 0.34 V, $$E_{Zn/Z{n^{2 + }}}^0$$ = + 0.76 V, $${{2.303RT} \over F} = 0.059\,V$$]
(A) Sublimation enthalpy
(B) Ionisation enthalpy
(C) Hydration enthalpy
(D) Electron gain enthalpy
The total number of above properties that affect the reduction potential is ____________ (Integer answer)
Cu(s) | Cu2+ (aq) (0.1 M) || Ag+(aq) (0.01 M) | Ag(s)
the cell potential E1 = 0.3095 V
For the cell
Cu(s) | Cu2+ (aq) (0.01 M) || Ag+(aq) (0.001 M) | Ag(s)
the cell potential = ____________ $$\times$$ 10$$-$$2 V. (Round off the nearest integer).
[Use : $${{2.303RT} \over F}$$ = 0.059]
Zn | Zn2+ (aq), (1M) || Fe3+ (aq), Fe2+ (aq) | Pt(s)
The fraction of total iron present as Fe3+ ion at the cell potential of 1.500 V is x $$\times$$ 10$$-$$2. The value of x is ______________. (Nearest integer)
(Given : $$E_{F{e^{3 + }}/F{e^{2 + }}}^0 = 0.77V$$, $$E_{Z{n^{2 + }}/Zn}^0 = - 0.76V$$)
$$C{u_{(s)}} + 2A{g^ + }(1 \times {10^{ - 3}}M) \to C{u^{2 + }}(0.250M) + 2A{g_{(s)}}$$
$$E_{cell}^\Theta = 2.97$$ V
Ecell for the above reaction is ______________ V. (Nearest integer)
[Given : log 2.5 = 0.3979, T = 298 K]
6OH$$-$$ + Cl$$-$$ $$\to$$ ClO3$$-$$ + 3H2O + 6e$$-$$
A current of xA has to be passed for 10h to produce 10.0g of potassium chlorate. The value of x is ____________. (Nearest integer)
(Molar mass of KClO3 = 122.6 g mol$$-$$1, F = 96500 C)
2Fe3+(aq) + 2I$$-$$(aq) $$ \to $$ 2Fe2+(aq) + I2(s)
the magnitude of the standard molar Gibbs free energy change, $$\Delta$$rG$$_m^o$$ = $$-$$ ___________ kJ (Round off to the Nearest Integer).
$$\left[ {\matrix{ {E_{F{e^{2 + }}/Fe(s)}^o = - 0.440V;} & {E_{F{e^{3 + }}/Fe(s)}^o = - 0.036V} \cr {E_{{I_2}/2{I^ - }}^o = 0.539V;} & {F = 96500C} \cr } } \right]$$
Zn|Zn2+(0.1 M)||Ag+ (0.01 M)|Ag
The value of x is _________. (Rounded off to the nearest integer)
[Given : $$E_{Z{n^{2 + }}/Zn}^\theta = - 0.76V;E_{A{g^{2 + }}/Ag}^\theta = + 0.80V;{{2.303RT} \over F} = 0.059$$]
$$MnO_4^ - + 8{H^ + } + 5{e^ - } \to M{n^{ + 2}} + 4{H_2}O,{E^o} = 1.51V$$.
The quantity of electricity required in Faraday to reduce five moles of $$MnO_4^ - $$ is ___________. (Integer answer)
[Given, $$E_{C{u^{2 + }}/Cu}^o = 0.34$$ V, $$E_{NO_3^ - /NO}^o = 0.96$$ V, $$E_{NO_3^ - /N{O_2}}^o = 0.79$$ V and at 298 K, $${{RT} \over F}$$(2.303) = 0.059]
6OH- + Cl- $$ \to $$ ClO3- + 3H2O + 6e-
If only 60% of the current is utilized in the reaction, the time (rounded to the nearest hour) required to produce 10 g of KClO3 using a current of 2 A is_________.
(Given : F = 96,500 C mol–1; molar mass of KCIO3 = 122 g mol–1)
3 electrons are transferred has a $$\Delta $$Gº of 17.37 kJ mol–1 at
25 oC. The value of Eo
cell (in V) is ______ × 10–2.
(1 F = 96,500 C mol–1)
Cr2O72- + 14H+ + 6e– $$ \to $$ 2Cr3+ + 7H2O
The amount of Cr3+ obtained was 0.104 g. The efficiency of the process(in%) is (Take : F = 96000 C, At. mass of chromium = 52) ______.
Pt(s) | H2 (g, 1 Bar) | HCl (aq., pH =1) | AgCl(s) | Ag(s).
The pH of aq. HCl required to stop the photoelectric current form K(w0 = 2.25 eV), all other conditions remaining the same, is _______ $$ \times $$ 10-2 (to the nearest integer).
Given, 2.303$${{RT} \over F}$$ = 0.06 V;
$$E_{AgCl|Ag|C{l^ - }}^0$$ = 0.22 V
2Cu+(aq) ⇌ Cu(s) + Cu2+(aq) at 298 K. ln K
(where K is the equilibrium constant) is
___________ × 10–1.
Given :
($$E_{C{u^{2 + }}/C{u^ + }}^0 = 0.16V$$
$$E_{C{u^ + }/Cu}^0 = 0.52V$$
$${{RT} \over F} = 0.025$$)
[Cu2+] = [Sn2+] = 1 M and 298K is :
Cu(s) + Sn2+(aq.) $$ \to $$ Cu2+(aq.) + Sn(s);
($$E_{S{n^{2 + }}|Sn}^0 = - 0.16\,V$$,
$$E_{C{u^{2 + }}|Cu}^0 = 0.34\,V$$)
Take F = 96500 C mol–1)
Sn(s) | Sn2+ (aq,1M)||Pb2+ (aq,1M)|Pb(s)
the ratio $${{\left[ {S{n^{2 + }}} \right]} \over {\left[ {P{b^{2 + }}} \right]}}$$ when this cell attains equilibrium is _________.
(Given $$E_{S{n^{2 + }}|Sn}^0 = - 0.14V$$,
$$E_{P{b^{2 + }}|Pb}^0 = - 0.13V$$, $${{2.303RT} \over F} = 0.06$$)
2H2O $$ \to $$ O2 + 4H$$ \oplus $$ + 4e– ; $$E_{red}^0$$ = 1.23 V
(R = 8.314 J mol–1 K–1 ; Temp = 298 k;
oxygen under std. atm. pressure of 1 bar)
MCQ (Single Correct Answer)
Given below are two statements :
1 M aqueous solutions of each of Cu(NO3)2, AgNO3, Hg2(NO3)2, Mg(NO3)2 are electrolysed using inert electrodes. Given: E0Ag+/Ag = 0.80 V, E0Hg22+/Hg = 0.79 V, E0Cu2+/Cu = 0.24 V and E0Mg2+/Mg = -2.37 V.
Statement (I) : With increasing voltage, the sequence of deposition of metals on the cathode will be Ag, Hg and Cu.
Statement (II) : Magnesium will not be deposited at the cathode instead oxygen gas will be evolved at the cathode.
In the light of the above statements, choose the most appropriate answer from the options given below :
On charging the lead storage battery, the oxidation state of lead changes from $x_1$ to $y_1$ at the anode and from $x_2$ to $y_2$ at the cathode. The values of $x_1, y_1, x_2, y_2$ are respectively :
The standard cell potential $\left(\mathrm{E}_{\text {cell }}^{\ominus}\right)$ of a fuel cell based on the oxidation of methanol in air that has been used to power television relay station is measured as 1.21 V . The standard half cell reduction potential for $\mathrm{O}_2\left(\mathrm{E}_{\mathrm{O}_2 / \mathrm{H}_2 \mathrm{O}}^{\circ}\right)$ is 1.229 V .
Choose the correct statement :
Match List - I with List - II :
| List - I (Applications) | List - II (Batteries/Cell) |
|---|---|
| (A) Transistors | (I) Anode - Zn/Hg; Cathode - HgO + C |
| (B) Hearing aids | (II) Hydrogen fuel cell |
| (C) Inverters | (III) Anode - Zn; Cathode - Carbon |
| (D) Apollo space ship | (IV) Anode - Pb; Cathode - Pb | PbO2 |
Choose the correct answer from the options given below :
$\mathrm{O}_2$ gas will be evolved as a product of electrolysis of :
(A) an aqueous solution of $\mathrm{AgNO}_3$ using silver electrodes.
(B) an aqueous solution of $\mathrm{AgNO}_3$ using platinum electrodes.
(C) a dilute solution of $\mathrm{H}_2 \mathrm{SO}_4$ using platinum electrodes.
(D) a high concentration solution of $\mathrm{H}_2 \mathrm{SO}_4$ using platinum electrodes.
Choose the correct answer from the options given below :
For a Mg | Mg2+ (aq) || Ag+ (aq) | Ag the correct Nernst Equation is :
The molar conductivity of a weak electrolyte when plotted against the square root of its concentration, which of the following is expected to be observed?
The standard reduction potential values of some of the p-block ions are given below. Predict the one with the strongest oxidising capacity.
Based on the data given below :
$$\begin{array}{ll} \mathrm{E}_{\mathrm{Cr}_2 \mathrm{O}_7^{2-} / \mathrm{Cr}^{3+}}^{\circ}=1.33 \mathrm{~V} & \mathrm{E}_{\mathrm{Cl}_2 / \mathrm{Cl}^{(-)}}^{\circ}=1.36 \mathrm{~V} \\ \mathrm{E}_{\mathrm{MnO}_4^{-} / \mathrm{Mn}^{2+}}^0=1.51 \mathrm{~V} & \mathrm{E}_{\mathrm{Cr}^{3+} / \mathrm{Cr}}^{\circ}=-0.74 \mathrm{~V} \end{array}$$
the strongest reducing agent is :
For the given cell
$$\mathrm{Fe}^{2+}(\mathrm{aq})+\mathrm{Ag}_{(\mathrm{aq})}^{+} \rightarrow \mathrm{Fe}^{3+}(\mathrm{aq})+\mathrm{Ag}_{(\mathrm{s})}$$
The standard cell potential of the above reaction is Given:
$$\begin{array}{lr} \mathrm{Ag}^{+}+\mathrm{e}^{-} \rightarrow \mathrm{Ag} & \mathrm{E}^\theta=\mathrm{xV} \\ \mathrm{Fe}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Fe} & \mathrm{E}^\theta=\mathrm{yV} \\ \mathrm{Fe}^{3+}+3 \mathrm{e}^{-} \rightarrow \mathrm{Fe} & \mathrm{E}^\theta=\mathrm{zV} \end{array}$$
Standard electrode potentials for a few half cells are mentioned below :
$$\begin{aligned} & \mathrm{E}_{\mathrm{Cu}^{2+} / \mathrm{Cu}}^{\circ}=0.34 \mathrm{~V}, \mathrm{E}_{\mathrm{Zn}^{2+} / \mathrm{Zn}}^{\circ}=-0.76 \mathrm{~V} \\ & \mathrm{E}_{\mathrm{Ag}^{+} / \mathrm{Ag}}^{\circ}=0.80 \mathrm{~V}, \mathrm{E}_{\mathrm{Mg}^{2+} / \mathrm{Mg}}^{\circ}=-2.37 \mathrm{~V} \end{aligned}$$
Which one of the following cells gives the most negative value of $\Delta \mathrm{G}^{\circ}$ ?
$$
\mathrm{FeO}_4^{2-} \xrightarrow{+2.0 \mathrm{~V}} \mathrm{Fe}^{3+} \xrightarrow{0.8 \mathrm{~V}} \mathrm{Fe}^{2+} \xrightarrow{-0.5 \mathrm{~V}} \mathrm{Fe}^0
$$
In the above diagram, the standard electrode potentials are given in volts (over the arrow).
The value of $\mathrm{E}_{\mathrm{FeO}_4^{2-} / \mathrm{Fe}^{2+}}$ is :
Given below are two statements :
Statement (I) : Corrosion is an electrochemical phenomenon in which pure metal acts as an anode and impure metal as a cathode.
Statement (II) : The rate of corrosion is more in alkaline medium than in acidic medium.
In the light of the above statements, choose the correct answer from the options given below :
Which of the following electrolyte can be used to obtain $\mathrm{H}_2 \mathrm{~S}_2 \mathrm{O}_8$ by the process of electrolysis ?
A solution of aluminium chloride is electrolysed for 30 minutes using a current of 2 A . The amount of the aluminium deposited at the cathode is __________ .
[Given : molar mass of aluminium and chlorine are $27 \mathrm{~g} \mathrm{~mol}^{-1}$ and $35.5 \mathrm{~g} \mathrm{~mol}^{-1}$ respectively. Faraday constant $\left.=96500 \mathrm{C} \mathrm{~mol}^{-1}\right]$
Match List I with List II
| LIST I (Cell) |
LIST II (Use/Property/Reaction) |
||
|---|---|---|---|
| A. | Leclanche cell | I. | Converts energy of combustion into electrical energy |
| B. | Ni - Cd cell | II. | Does not involve any ion in solution and is used in hearing aids |
| C. | Fuel cell | III. | Rechargeable |
| D. | Mercury cell | IV. | Reaction at anode $$\mathrm{Zn} \rightarrow \mathrm{Zn}^{2+}+2 \mathrm{e}^{-}$$ |
Choose the correct answer from the options given below :
Which out of the following is a correct equation to show change in molar conductivity with respect to concentration for a weak electrolyte, if the symbols carry their usual meaning :
The molar conductivity for electrolytes $$A$$ and $$B$$ are plotted against $$C^{3 / 2}$$ as shown below. Electrolytes $$A$$ and $$B$$ respectively are:

The emf of cell $$\mathrm{Tl}\left|\underset{(0.001 \mathrm{M})}{\mathrm{Tl}^{+}}\right| \underset{(0.01 \mathrm{M})}{\mathrm{Cu}^{2+}} \mid \mathrm{Cu}$$ is $$0.83 \mathrm{~V}$$ at $$298 \mathrm{~K}$$. It could be increased by :
The reaction;
$$\frac{1}{2} \mathrm{H}_{2(\mathrm{~g})}+\mathrm{AgCl}_{(\mathrm{s})} \rightarrow \mathrm{H}_{(\mathrm{aq})}^{+}+\mathrm{Cl}_{(\mathrm{aq})}^{-}+\mathrm{Ag}_{(\mathrm{s})}$$
occurs in which of the following galvanic cell :
Given below are two statements :
Statement (I) : Fusion of $$\mathrm{MnO}_2$$ with $$\mathrm{KOH}$$ and an oxidising agent gives dark green $$\mathrm{K}_2 \mathrm{MnO}_4$$.
Statement (II) : Manganate ion on electrolytic oxidation in alkaline medium gives permanganate ion.
In the light of the above statements, choose the correct answer from the options given below :
How can an electrochemical cell be converted into an electrolytic cell ?
A conductivity cell with two electrodes (dark side) are half filled with infinitely dilute aqueous solution of a weak electrolyte. If volume is doubled by adding more water at constant temperature, the molar conductivity of the cell will -

The quantity of silver deposited when one coulomb charge is passed through $$\mathrm{AgNO}_3$$ solution :
For the electro chemical cell
$$\mathrm{M}\left|\mathrm{M}^{2+}\right||\mathrm{X}| \mathrm{X}^{2-}$$
If $$\mathrm{E}_{\left(\mathrm{M}^{2+} / \mathrm{M}\right)}^0=0.46 \mathrm{~V}$$ and $$\mathrm{E}_{\left(\mathrm{x} / \mathrm{x}^{2-}\right)}^0=0.34 \mathrm{~V}$$.
Which of the following is correct?
Molar ionic conductivities of divalent cation and anion are $$57 \mathrm{~S~cm}^2 \mathrm{~mol}^{-1}$$ and $$73 \mathrm{~S~cm}^2 \mathrm{~mol}^{-1}$$ respectively. The molar conductivity of solution of an electrolyte with the above cation and anion will be:
The reaction at cathode in the cells commonly used in clocks involves.
Fuel cell, using hydrogen and oxygen as fuels,
A. has been used in spaceship
B. has as efficiency of $$40 \%$$ to produce electricity
C. uses aluminum as catalysts
D. is eco-friendly
E. is actually a type of Galvanic cell only
Choose the correct answer from the options given below:
For a strong electrolyte, a plot of molar conductivity against (concentration) $${ }^{1 / 2}$$ is a straight line, with a negative slope, the correct unit for the slope is
One of the commonly used electrode is calomel electrode. Under which of the following categories, calomel electrode comes?
What pressure (bar) of $$\mathrm{H}_2$$ would be required to make emf of hydrogen electrode zero in pure water at $$25^{\circ} \mathrm{C}$$ ?
Identify the factor from the following that does not affect electrolytic conductance of a solution.
Alkaline oxidative fusion of $$\mathrm{MnO}_2$$ gives "A" which on electrolytic oxidation in alkaline solution produces B. A and B respectively are
Reduction potential of ions are given below:
$$\begin{array}{ccc} \mathrm{ClO}_4^{-} & \mathrm{IO}_4^{-} & \mathrm{BrO}_4^{-} \\ \mathrm{E}^{\circ}=1.19 \mathrm{~V} & \mathrm{E}^{\circ}=1.65 \mathrm{~V} & \mathrm{E}^{\circ}=1.74 \mathrm{~V} \end{array}$$
The correct order of their oxidising power is :
Which of the following statements is not correct about rusting of iron?
For lead storage battery pick the correct statements
A. During charging of battery, $$\mathrm{PbSO}_{4}$$ on anode is converted into $$\mathrm{PbO}_{2}$$
B. During charging of battery, $$\mathrm{PbSO}_{4}$$ on cathode is converted into $$\mathrm{PbO}_{2}$$
C. Lead storage battery consists of grid of lead packed with $$\mathrm{PbO}_{2}$$ as anode
D. Lead storage battery has $$\sim 38 \%$$ solution of sulphuric acid as an electrolyte
Choose the correct answer from the options given below:
The reaction
$$\frac{1}{2} \mathrm{H}_{2}(\mathrm{~g})+\mathrm{AgCl}(\mathrm{s}) \rightleftharpoons \mathrm{H}^{+}(\mathrm{aq})+\mathrm{Cl}^{-}(\mathrm{aq})+\mathrm{Ag}(\mathrm{s})$$
occurs in which of the given galvanic cell.
The standard electrode potential of $$\mathrm{M}^{+} / \mathrm{M}$$ in aqueous solution does not depend on
Given below are two statements : one is labelled as Assertion (A) and the other is labelled as Reason (R)
Assertion (A) : An aqueous solution of $$\mathrm{KOH}$$ when used for volumetric analysis, its concentration should be checked before the use.
Reason (R) : On aging, $$\mathrm{KOH}$$ solution absorbs atmospheric $$\mathrm{CO}_{2}$$.
In the light of the above statements, choose the correct answer from the options given below :
Which one of the following statements is correct for electrolysis of brine solution?
The standard electrode potential $$\mathrm{(M^{3+}/M^{2+})}$$ for V, Cr, Mn & Co are $$-$$0.26 V, $$-$$0.41 V, + 1.57 V and + 1.97 V, respectively. The metal ions which can liberate $$\mathrm{H_2}$$ from a dilute acid are :
Choose the correct representation of conductometric titration of benzoic acid vs sodium hydroxide.
Match List - I with List - II.
| List - I | List - II | ||
|---|---|---|---|
| (A) | $$Cd(s) + 2Ni{(OH)_3}(s) \to CdO(s) + 2Ni{(OH)_2}(s) + {H_2}O(l)$$ | (I) | Primary battery |
| (B) | $$Zn(Hg) + HgO(s) \to ZnO(s) + Hg(l)$$ | (II) | Discharging of secondary battery |
| (C) | $$2PbS{O_4}(s) + 2{H_2}O(l) \to Pb(s) + Pb{O_2}(s) + 2{H_2}S{O_4}(aq)$$ | (III) | Fuel cell |
| (D) | $$2{H_2}(g) + {O_2}(g) \to 2{H_2}O(l)$$ | (IV) | Charging of secondary battery |
Choose the correct answer from the options given below:
Given below are two statements :
Statement I : For KI, molar conductivity increases steeply with dilution
Statement II : For carbonic acid, molar conductivity increases slowly with dilution
In the light of the above statements, choose the correct answer from the options given below :
The molar conductivity of a conductivity cell filled with 10 moles of 20 mL NaCl solution is $${\Lambda _{m1}}$$ and that of 20 moles another identical cell heaving 80 mL NaCl solution is $${\Lambda _{m2}}$$. The conductivities exhibited by these two cells are same. The relationship between $${\Lambda _{m2}}$$ and $${\Lambda _{m1}}$$ is
In which of the following half cells, electrochemical reaction is pH dependent?
In 3d series, the metal having the highest M2+/M standard electrode potential is :
The $${\left( {{{\partial E} \over {\partial T}}} \right)_P}$$ of different types of half cells are as follows:
| A | B | C | D |
|---|---|---|---|
| $$1 \times {10^{ - 4}}$$ | $$2 \times {10^{ - 4}}$$ | $$0.1 \times {10^{ - 4}}$$ | $$0.2 \times {10^{ - 4}}$$ |
(Where E is the electromotive force)
Which of the above half cells would be preferred to be used as reference electrode?
The correct order of reduction potentials of the following pairs is
A. Cl2/Cl$$-$$
B. I2/I$$-$$
C. Ag+/Ag
D. Na+/Na
E. Li+/Li
Choose the correct answer from the options given below.
| List - I (Parmeter) |
List - II (Unit) |
||
|---|---|---|---|
| (a) | Cell constant | (i) | $$S\,c{m^2}mo{l^{ - 1}}$$ |
| (b) | Molar conductivity | (ii) | Dimensionless |
| (c) | Conductivity | (iii) | $${m^{ - 1}}$$ |
| (d) | Degree of dissociation of electrolyte | (iv) | $${\Omega ^{ - 1}}{m^{ - 1}}$$ |
Choose the most appropriate answer from the options given below :
Statement I : The limiting molar conductivity of KCl (strong electrolyte) is higher compared to that of CH3COOH (weak electrolyte).
Statement II : Molar conductivity decreases with decrease in concentration of electrolyte.
In the light of the above statements, choose the most appropriate answer from the options given below :
Cu(s) | Cu2+(C1M) || Cu2+(C2M) | Cu(s)
change in Gibbs energy ($$\Delta $$G) is negative, if :
The electrolyte X is :
[ $$E_{A{g^ + }/Ag}^0$$ = 0.80 V, $$E_{A{u^ + }/Au}^0$$ = 1.69 V ]
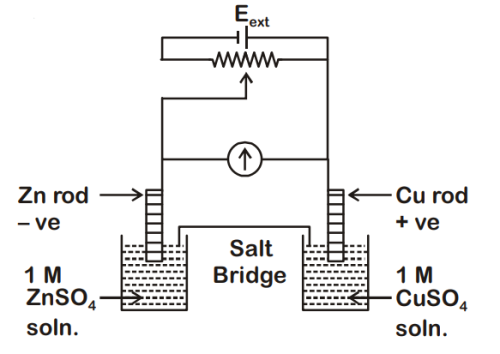
$$E_{C{u^{2 + }}|Cu}^0$$ = +0.34 V
$$E_{Z{n^{2 + }}|Zn}^0$$ = -0.76 V
Identify the incorrect statement from the option below for the above cell :
CO3+ + e– $$ \to $$ CO2+ ; Eo = + 1.81 V
Pb4+ + 2e– $$ \to $$ Pb2+ ; Eo = + 1.67 V
Ce4+ + e– $$ \to $$ Ce3+ ; Eo = + 1.61 V
Bi3+ + 3e– $$ \to $$ Bi ; Eo = + 0.20 V
Oxidizing power of the species will increase in the order :
S1 : Conductivity always increases with decrease in the concentration of electrolyte.
S2 : Molar conductivity always increases with decrease in the concentration of electrolyte.
The correct option among the following is :
Zn(s) + Cu2+ (aq) $$ \to $$ Zn2+ (aq) + Cu (s),
E° = 2 V at 298 K
(Faraday's constant, F = 96000 C mol–1)
Fe2+(aq) + Ag+(aq) $$ \to $$ Fe3+(aq) + Ag (s)
Given that
$$E_{A{g^ + }/Ag}^o = xV$$
$$E_{Fe^{2+ }/Fe}^o = yV$$
$$E_{Fe^{3+ }/Fe}^o = zV$$
$${E^\Theta }_{{S_2}O_8^{2 - }/SO_4^{2 - }} = 2.05\,V$$
$${E^\Theta }_{B{r_2}/B{r^ - }} = 1.09\,V$$
$${E^\Theta }_{A{u^{3 + }}/Au} = 1.4\,V$$
The strongest oxidizing agent is :
5 $$ \times $$ 10–5 S cm–1, degree of dissociation of HA is -
The cell reaction is
Zn(s) + Cu2+ (aq) $$\buildrel \, \over \longrightarrow $$ Zn2+ (aq) + Cu(s)
The standard reaction enthalpy ($$\Delta $$rH$${^o }$$) at 300 K in kJ mol–1 is, [Use R = 8 JK–1 mol–1 and F = 96,000C mol–1]
KC of the reaction :
Cu(s) + 2Ag+ (aq) $$ \to $$ Cu2+ (aq) + 2Ag(s) is
10 $$ \times $$ 1015, calculate the E$$_{cell}^0$$ of this reaciton at 298 K
[2.303 $${{RT} \over F}$$ at 298 K = 0.059V]
| Mx+ (aq)/M(s) | Au3+(aq)/Au(s) | Ag+(aq)/Ag(s) | Fe3+(aq)/Fe2+ (aq) | Fe2+(aq)/Fe(s) |
|---|---|---|---|---|
| E0Mx+/M/(V) | 1.40 | 0.80 | 0.77 | $$-$$0.44 |
If $$E_{z{n^{2 + }}/zn}^0$$ = $$-$$ 0.76 V, which cathode will give maximum value of Eocell per electron transferred?
Pt$$\left| {\left( s \right)} \right|$$H2(g, 1 bar)$$\left| {HCl\left( {aq} \right)} \right|$$AgCl$$\left| {\left( s \right)} \right|$$Ag(s)|Pt(s)
the cell potential is 0.92 V when a 10–6 molal HCl solution is used. The standard electrode potential of (AgCl/ AgCl– ) electrode is :
$$\left\{ {} \right.$$Given, $${{2.303RT} \over F} = 0.06V$$ at $$\left. {298} \right\}$$
Zn2+ + 2e– $$ \to $$ Zn(s) ; Eo = – 0.76 V
Ca2+ + 2e– $$ \to $$ Ca(s); Eo = –2.87 V
Mg2+ + 2e– $$ \to $$ Mg(s) ; Eo = – 2.36 V
Ni2 + 2e– $$ \to $$ Ni(s) ; Eo = – 0.25
The reducing power of the metals increases in the order :
Zn(s) + Cu2+ (aq) $$\rightleftharpoons$$ Zn2+(aq) + Cu(s)
at 300 K is approximately,
(R = 8 JK$$-$$1mol$$-$$1, F = 96000 C mol$$-$$1)
(Atomic weight of B = 10.8 u)
The emf of the cell is found to be 0.421 volt at 298 K. The standard potential of half reaction M3+ + 3e−$$ \to $$ M at 298 K will be :
(Given $$E_{A{g^ + }\,/\,Ag}^ - $$ at 298 K = 0.80 Volt)
Given that :
Fe2+ + 2e$$-$$ $$ \to $$ Fe; $$E_{F{e^{2 + }}/Fe}^o$$ = $$-$$0.47 V
Fe3+ + e$$-$$ $$ \to $$ Fe2+; $$E_{F{e^{3 + }}/F{e^{2 + }}}^o$$ = +0.77 V
| Element | M3+ /M | M+ /M |
|---|---|---|
| A1 | -1.66 | + 0.55 |
| T1 | +1.26 | - 0.34 |
Based on these data, which of the following statements is correct ?
$$E_{C{l_2}/C{l^ - }}^o$$ = 1.36 V, $$E_{C{r^{3 + }}/Cr}^o$$ = - 0.74 V
$$E_{C{r_2}{O_7}^{2 - }/C{r^{3 + }}}^o$$ = 1.33 V, $$E_{Mn{O_4}^ - /Mn ^{2+}}^o$$ = 1.51 V
Among the following, the strongest reducing agent is :
Mn2+ + 2e- $$\to$$ Mn; Eo = -1.18 V
2(Mn3+ + e- $$\to$$ Mn2+); Eo = +1.51 V
The Eo for 3Mn2+ $$\to$$ Mn + 2Mn3+ will be :
$$E_{C{r^{2 + }}/Cr}^o$$ = -0.74 V; $$E_{MnO_4^ - /M{n^{2 + }}}^o$$ = 1.51 V
$$E_{C{r_2}O_7^{2 - }/C{r^{3 + }}}^o$$ = 1.33 V; $$E_{Cl/C{l^ - }}^o$$ = 1.36 V
Based on the data given above, strongest oxidising agent will be :
X + Y2+ $$\to$$ X2+ + Y will be spontaneous when :
$${2 \over 3}A{l_2}{O_3}$$ $$\to$$ $${4 \over 3}Al + {O_2}$$, $${\Delta _r}G$$ = + 966 kJ mol–1
The potential difference needed for electrolytic reduction of Al2O3 at 500oC is at least :
The value of standard electrode potential for the change,
Fe3+ (aq) + e- $$\to$$ Fe2+ (aq) will be
CH3OH(l) + 3/2O2 $$\to$$ CO2 (g) + 2H2O (l)
At 298K standard Gibb’s energies of formation for CH3OH(l), H2O(l) and CO2 (g) are -166.2, -237.2 and -394.4 kJ mol−1 respectively. If standard enthalpy of combustion of methanol is -726 kJ mol−1, efficiency of the fuel cell will be
$$ \wedge _{C{H_3}COONa}^o$$ = 91.0 S cm2/equiv
$$ \wedge _{HCl}^o$$ = 426.2 S cm2/equiv
What additional information/quantity one needs to calculate $$ \wedge ^o$$ of an aqueous solution of acetic acid?
Ag + I- $$\to$$ AgI + e- , Eo = 0.152 V
Ag $$\to$$ Ag+ + e-, Eo = -0.800 V
What is the value of log Ksp for AgI? (2.303 RT/F = 0.059 V)
| Electrolyte: | KCl | KNO3 | HCl | NaOAc | NaCl |
|---|---|---|---|---|---|
| $${ \wedge ^\infty }(Sc{m^2}mo{l^{ - 1}}):$$ |
149.9 | 145 | 426.2 | 91 | 126.5 |
$$E_{F{e^{3 + }}/F{e^{2 + }}}^o$$ = 0.77 V;
$$E_{S{n^{2 + }}/S{n}}^o$$ = -0.14 V
Under standard conditions the potential for the reaction
Sn(s) + 2Fe3+(aq) $$\to$$ 2Fe2+(aq) + Sn2+(aq) is :
$$\eqalign{ & Pt({H_2})|{H^ + }(aq)|Pt({H_2}) \cr & \,\,\,\,\,{p_1}\,\,\,\,\,\,\,\,\,\,\,\,\,\,1M\,\,\,\,\,\,\,\,\,\,\,\,{p_2} \cr} $$

$$ \begin{aligned} \mathrm{Ag}^{+}+\mathrm{e}^{-} & \longrightarrow \mathrm{Ag}; E^{\circ}=x \\\\ \mathrm{Cu}^{2+}+2 e^{-} & \longrightarrow \mathrm{Cu}{;} E^{\circ}=y \end{aligned} $$
$$ E^{\circ} \text { cell is } $$ :