Thermodynamics · Chemistry · JEE Main
Numerical
Resonance in $\mathrm{X}_2 \mathrm{Y}$ can be represented as

The enthalpy of formation of $X_2Y$ $ \left(X = X(g) + \frac{1}{2} Y = Y(g) \rightarrow X_2Y(g) \right) $ is 80 kJ mol$^{-1}$. The magnitude of resonance energy of $X_2Y$ is __ kJ mol$^{-1}$ (nearest integer value).
Given: Bond energies of $X \equiv X$, $X = X$, $Y = Y$ and $X = Y$ are 940, 410, 500, and 602 kJ mol$^{-1}$ respectively.
valence $X$: 3, $Y$: 2
A perfect gas ( 0.1 mol ) having $\overline{\mathrm{C}}_v=1.50 \mathrm{R}$ (independent of temperature) undergoes the above transformation from point 1 to point 4. If each step is reversible, the total work done (w) while going from point 1 to point 4 is $(-)$___________$J$ (nearest integer)
[Given: $\mathrm{R}=0.082 \mathrm{~L} \mathrm{~atm} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}$ ]

Given :
$$ \begin{aligned} & \left.\Delta \mathrm{H}^{\ominus}{ }_{\text {sub }}[\mathrm{C} \text { (graphite })\right]=710 \mathrm{~kJ} \mathrm{~mol}^{-1} \\ & \Delta_{\mathrm{C}-\mathrm{H}} \mathrm{H}^{\ominus}=414 \mathrm{~kJ} \mathrm{~mol}^{-1} \\ & \Delta_{\mathrm{H}-\mathrm{H}} \mathrm{H}^{\ominus}=436 \mathrm{~kJ} \mathrm{~mol}^{-1} \\ & \Delta_{\mathrm{C}}=\mathrm{C} \mathrm{H}^{\ominus}=611 \mathrm{~kJ} \mathrm{~mol}^{-1} \end{aligned} $$
The $\Delta \mathrm{H}_{\mathrm{f}} \ominus$ for $\mathrm{CH}_2=\mathrm{CH}_2$ is_________ $\mathrm{kJ} \mathrm{mol}^{-1}$ (nearest integer value)
Consider the following data :
Heat of formation of $\mathrm{CO}_2(\mathrm{g})=-393.5 \mathrm{~kJ} \mathrm{~mol}{ }^{-1}$
Heat of formation of $\mathrm{H}_2 \mathrm{O}(\mathrm{l})=-286.0 \mathrm{~kJ} \mathrm{~mol}{ }^{-1}$
Heat of combustion of benzene $=-3267.0 \mathrm{~kJ} \mathrm{~mol}^{-1}$
The heat of formation of benzene is __________ $\mathrm{kJ} \mathrm{mol}^{-1}$. (Nearest integer)
The formation enthalpies, $\Delta \mathrm{H}_{\mathrm{f}}^{\ominus}$ for $\mathrm{H}_{(\mathrm{g})}$ and $\mathrm{O}_{(\mathrm{g})}$ are 220.0 and $250.0 \mathrm{~kJ} \mathrm{~mol}^{-1}$, respectively, at 298.15 K , and $\Delta \mathrm{H}_{\mathrm{f}}^{\ominus}$ for $\mathrm{H}_2 \mathrm{O}_{(\mathrm{g})}$ is $-242.0 \mathrm{~kJ} \mathrm{~mol}^{-1}$ at the same temperature. The average bond enthalpy of the $\mathrm{O}-\mathrm{H}$ bond in water at 298.15 K is _______ $\mathrm{kJ} \mathrm{~mol}^{-1}$ (nearest integer).
Standard entropies of $\mathrm{X}_2, \mathrm{Y}_2$ and $\mathrm{XY}_5$ are 70, 50 and $110 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}$ respectively. The temperature in Kelvin at which the reaction
$$\frac{1}{2} \mathrm{X}_2+\frac{5}{2} \mathrm{Y}_2 \rightleftharpoons \mathrm{XY}_5 \Delta \mathrm{H}^{\ominus}=-35 \mathrm{~kJ} \mathrm{~mol}^{-1}$$
will be at equilibrium is __________ (Nearest integer)
The bond dissociation enthalpy of $\mathrm{X}_2 \Delta \mathrm{H}_{\text {bond }}^{\circ}$ calculated from the given data is ___________ $\mathrm{kJ} \mathrm{mol}^{-1}$. (Nearest integer)
$$\begin{aligned} & \mathrm{M}^{+} \mathrm{X}^{-}(\mathrm{s}) \rightarrow \mathrm{M}^{+}(\mathrm{g})+\mathrm{X}^{-}(\mathrm{g}) \Delta \mathrm{H}_{\text {lattice }}^{\circ}=800 \mathrm{~kJ} \mathrm{~mol}^{-1} \\ & \mathrm{M}(\mathrm{~s}) \rightarrow \mathrm{M}(\mathrm{~g}) \Delta \mathrm{H}_{\text {sub }}^{\circ}=100 \mathrm{~kJ} \mathrm{~mol}^{-1} \end{aligned}$$
$$\mathrm{M}(\mathrm{~g}) \rightarrow \mathrm{M}^{+}(\mathrm{g})+\mathrm{e}^{-}(\mathrm{g}) \Delta \mathrm{H}_{\mathrm{i}}^{\circ}=500 \mathrm{~kJ} \mathrm{~mol}^{-1}$$
$$\mathrm{X}(\mathrm{~g})+\mathrm{e}^{-}(\mathrm{g}) \rightarrow \mathrm{X}^{-}(\mathrm{g}) \Delta \mathrm{H}_{\mathrm{eg}}^{\circ}=-300 \mathrm{~kJ} \mathrm{~mol}^{-1}$$
$$\mathrm{M}(\mathrm{~s})+\frac{1}{2} \mathrm{X}_2(\mathrm{~g}) \rightarrow \mathrm{M}^{+} \mathrm{X}^{-}(\mathrm{s}) \Delta \mathrm{H}_f^{\circ}=-400 \mathrm{~kJ} \mathrm{~mol}^{-1}$$
[Given : $\mathrm{M}^{+} \mathrm{X}^{-}$is a pure ionic compound and X forms a diatomic molecule $\mathrm{X}_2$ in gaseous state]
The standard enthalpy and standard entropy of decomposition of $\mathrm{N}_2 \mathrm{O}_4$ to $\mathrm{NO}_2$ are $55.0 \mathrm{~kJ} \mathrm{~mol}^{-1}$ and $175.0 \mathrm{~J} / \mathrm{K} / \mathrm{mol}$ respectively. The standard free energy change for this reaction at $25^{\circ} \mathrm{C}$ in J $\mathrm{mol}^{-1}$ is ________ (Nearest integer)
Consider the following cases of standard enthalpy of reaction $\left(\Delta \mathrm{H}_{\mathrm{r}}^{\circ}\right.$ in $\left.\mathrm{kJ} \mathrm{mol}^{-1}\right)$
$$\begin{aligned} & \mathrm{C}_2 \mathrm{H}_6(\mathrm{~g})+\frac{7}{2} \mathrm{O}_2(\mathrm{~g}) \rightarrow 2 \mathrm{CO}_2(\mathrm{~g})+3 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \Delta \mathrm{H}_1^{\circ}=-1550 \\ & \mathrm{C}(\text { graphite })+\mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{CO}_2(\mathrm{~g}) \Delta \mathrm{H}_2^{\circ}=-393.5 \\ & \mathrm{H}_2(\mathrm{~g})+\frac{1}{2} \mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \Delta \mathrm{H}_3^{\circ}=-286 \end{aligned}$$
The magnitude of $\Delta \mathrm{H}_{f \mathrm{C}_2 \mathrm{H}_6(\mathrm{~g})}^{\circ}$ is ____________ $\mathrm{kJ} \mathrm{mol}^{-1}$ (Nearest integer).
When $$\Delta \mathrm{H}_{\mathrm{vap}}=30 \mathrm{~kJ} / \mathrm{mol}$$ and $$\Delta \mathrm{S}_{\mathrm{vap}}=75 \mathrm{~J} \mathrm{~mol}^{-1} \mathrm{~K}^{-1}$$, then the temperature of vapour, at one atmosphere is _________ K.
When equal volume of $$1 \mathrm{~M} \mathrm{~HCl}$$ and $$1 \mathrm{~M} \mathrm{~H}_2 \mathrm{SO}_4$$ are separately neutralised by excess volume of $$1 \mathrm{M}$$ $$\mathrm{NaOH}$$ solution. $$x$$ and $$y \mathrm{~kJ}$$ of heat is liberated respectively. The value of $$y / x$$ is __________.
The heat of solution of anhydrous $$\mathrm{CuSO}_4$$ and $$\mathrm{CuSO}_4 \cdot 5 \mathrm{H}_2 \mathrm{O}$$ are $$-70 \mathrm{~kJ} \mathrm{~mol}^{-1}$$ and $$+12 \mathrm{~kJ} \mathrm{~mol}^{-1}$$ respectively.
The heat of hydration of $$\mathrm{CuSO}_4$$ to $$\mathrm{CuSO}_4 \cdot 5 \mathrm{H}_2 \mathrm{O}$$ is $$-x \mathrm{~kJ}$$. The value of $$x$$ is ________. (nearest integer).
$$\Delta_{\text {vap }} \mathrm{H}^{\ominus}$$ for water is $$+40.79 \mathrm{~kJ} \mathrm{~mol}^{-1}$$ at 1 bar and $$100^{\circ} \mathrm{C}$$. Change in internal energy for this vapourisation under same condition is ________ $$\mathrm{kJ} \mathrm{~mol}^{-1}$$. (Integer answer) (Given $$\mathrm{R}=8.3 \mathrm{~JK}^{-1} \mathrm{~mol}^{-1}$$)

Consider the figure provided.
$$1 \mathrm{~mol}$$ of an ideal gas is kept in a cylinder, fitted with a piston, at the position A, at $$18^{\circ} \mathrm{C}$$. If the piston is moved to position $$\mathrm{B}$$, keeping the temperature unchanged, then '$$\mathrm{x}$$' $$\mathrm{L}$$ atm work is done in this reversible process.
$$\mathrm{x}=$$ ________ $$\mathrm{L}$$ atm. (nearest integer)
[Given : Absolute temperature $$={ }^{\circ} \mathrm{C}+273.15, \mathrm{R}=0.08206 \mathrm{~L} \mathrm{~atm} \mathrm{~mol}{ }^{-1} \mathrm{~K}^{-1}$$]
For the reaction at $$298 \mathrm{~K}, 2 \mathrm{~A}+\mathrm{B} \rightarrow \mathrm{C}, \Delta \mathrm{H}=400 \mathrm{~kJ} \mathrm{~mol}^{-1}$$ and $$\Delta S=0.2 \mathrm{~kJ} \mathrm{~mol}^{-1} \mathrm{~K}^{-1}$$. The reaction will become spontaneous above __________ $$\mathrm{K}$$.
An ideal gas, $$\overline{\mathrm{C}}_{\mathrm{v}}=\frac{5}{2} \mathrm{R}$$, is expanded adiabatically against a constant pressure of 1 atm untill it doubles in volume. If the initial temperature and pressure is $$298 \mathrm{~K}$$ and $$5 \mathrm{~atm}$$, respectively then the final temperature is _________ $$\mathrm{K}$$ (nearest integer).
[$$\overline{\mathrm{c}}_{\mathrm{v}}$$ is the molar heat capacity at constant volume]
Combustion of 1 mole of benzene is expressed at
$$\mathrm{C}_6 \mathrm{H}_6(\mathrm{l})+\frac{15}{2} \mathrm{O}_2(\mathrm{~g}) \rightarrow 6 \mathrm{CO}_2(\mathrm{~g})+3 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \text {. }$$
The standard enthalpy of combustion of $$2 \mathrm{~mol}$$ of benzene is $$-^{\prime} x^{\prime} \mathrm{kJ}$$. $$x=$$ __________.
Given :
1. standard Enthalpy of formation of $$1 \mathrm{~mol}$$ of $$\mathrm{C}_6 \mathrm{H}_6(\mathrm{l})$$, for the reaction $$6 \mathrm{C}$$ (graphite) $$+3 \mathrm{H}_2(\mathrm{g}) \rightarrow \mathrm{C}_6 \mathrm{H}_6(\mathrm{l})$$ is $$48.5 \mathrm{~kJ} \mathrm{~mol}^{-1}$$.
2. Standard Enthalpy of formation of $$1 \mathrm{~mol}$$ of $$\mathrm{CO}_2(\mathrm{g})$$, for the reaction $$\mathrm{C}$$ (graphite) $$+\mathrm{O}_2(\mathrm{g}) \rightarrow \mathrm{CO}_2(\mathrm{g})$$ is $$-393.5 \mathrm{~kJ} \mathrm{~mol}^{-1}$$.
3. Standard and Enthalpy of formation of $$1 \mathrm{~mol}$$ of $$\mathrm{H}_2 \mathrm{O}(\mathrm{l})$$, for the reaction $$\mathrm{H}_2(\mathrm{g})+\frac{1}{2} \mathrm{O}_2(\mathrm{g}) \rightarrow \mathrm{H}_2 \mathrm{O}(\mathrm{l})$$ is $$-286 \mathrm{~kJ} \mathrm{~mol}^{-1}$$.
The heat of combustion of solid benzoic acid at constant volume is $$-321.30 \mathrm{~kJ}$$ at $$27^{\circ} \mathrm{C}$$. The heat of combustion at constant pressure is $$(-321.30-x \mathrm{R}) \mathrm{~kJ}$$, the value of $$x$$ is __________.
Three moles of an ideal gas are compressed isothermally from $$60 \mathrm{~L}$$ to $$20 \mathrm{~L}$$ using constant pressure of $$5 \mathrm{~atm}$$. Heat exchange $$\mathrm{Q}$$ for the compression is - _________ Lit. atm.
The enthalpy of formation of ethane $$(\mathrm{C}_2 \mathrm{H}_6)$$ from ethylene by addition of hydrogen where the bond-energies of $$\mathrm{C}-\mathrm{H}, \mathrm{C}-\mathrm{C}, \mathrm{C}=\mathrm{C}, \mathrm{H}-\mathrm{H}$$ are $$414 \mathrm{~kJ}, 347 \mathrm{~kJ}, 615 \mathrm{~kJ}$$ and $$435 \mathrm{~kJ}$$ respectively is $$-$$ __________ $$\mathrm{kJ}$$
(Given $\mathrm{R}=8.314 \mathrm{JK}^{-1} \mathrm{~mol}^{-1}$ )
If 5 moles of an ideal gas expands from $$10 \mathrm{~L}$$ to a volume of $$100 \mathrm{~L}$$ at $$300 \mathrm{~K}$$ under isothermal and reversible condition then work, $$\mathrm{w}$$, is $$-x \mathrm{~J}$$. The value of $$x$$ is __________.
(Given R = 8.314 J K$$^{-1}$$ mol$$^{-1}$$)
Consider the following reaction at $$298 \mathrm{~K} \cdot \frac{3}{2} \mathrm{O}_{2(g)} \rightleftharpoons \mathrm{O}_{3(g)} \cdot \mathrm{K}_{\mathrm{P}}=2.47 \times 10^{-29}$$. $$\Delta_r G^{\ominus}$$ for the reaction is _________ $$\mathrm{kJ}$$. (Given $$\mathrm{R}=8.314 \mathrm{~JK}^{-1} \mathrm{~mol}^{-1}$$)
Two reactions are given below:
$$\begin{aligned} & 2 \mathrm{Fe}_{(\mathrm{s})}+\frac{3}{2} \mathrm{O}_{2(\mathrm{~g})} \rightarrow \mathrm{Fe}_2 \mathrm{O}_{3(\mathrm{~s})}, \Delta \mathrm{H}^{\circ}=-822 \mathrm{~kJ} / \mathrm{mol} \\ & \mathrm{C}_{(\mathrm{s})}+\frac{1}{2} \mathrm{O}_{2(\mathrm{~g})} \rightarrow \mathrm{CO}_{(\mathrm{g})}, \Delta \mathrm{H}^{\circ}=-110 \mathrm{~kJ} / \mathrm{mol} \end{aligned}$$
Then enthalpy change for following reaction $$3 \mathrm{C}_{(\mathrm{s})}+\mathrm{Fe}_2 \mathrm{O}_{3(\mathrm{~s})} \rightarrow 2 \mathrm{Fe}_{(\mathrm{s})}+3 \mathrm{CO}_{(\mathrm{g})}$$ is _______ $$\mathrm{kJ} / \mathrm{mol}$$.

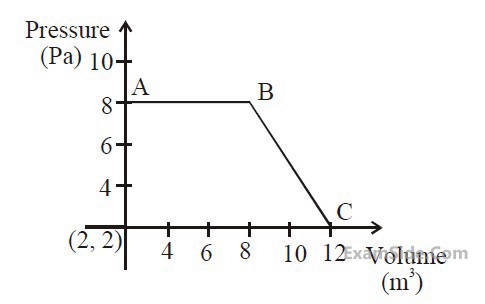
An ideal gas undergoes a cyclic transformation starting from the point A and coming back to the same point by tracing the path $$\mathrm{A} \rightarrow \mathrm{B} \rightarrow \mathrm{C} \rightarrow \mathrm{A}$$ as shown in the diagram above. The total work done in the process is __________ J.
Standard enthalpy of vapourisation for $$\mathrm{CCl}_4$$ is $$30.5 \mathrm{~kJ} \mathrm{~mol}^{-1}$$. Heat required for vapourisation of $$284 \mathrm{~g}$$ of $$\mathrm{CCl}_4$$ at constant temperature is ________ $$\mathrm{kJ}$$.
(Given molar mass in $$\mathrm{g} \mathrm{mol}^{-1} ; \mathrm{C}=12, \mathrm{Cl}=35.5$$)
For a certain thermochemical reaction $$\mathrm{M} \rightarrow \mathrm{N}$$ at $$\mathrm{T}=400 \mathrm{~K}, \Delta \mathrm{H}^{\ominus}=77.2 \mathrm{~kJ} \mathrm{~mol}^{-1}, \Delta \mathrm{S}=122 \mathrm{~JK}^{-1}, \log$$ equilibrium constant $$(\log K)$$ is __________ $$\times 10^{-1}$$.
If three moles of an ideal gas at $$300 \mathrm{~K}$$ expand isothermally from $$30 \mathrm{~dm}^3$$ to $$45 \mathrm{~dm}^3$$ against a constant opposing pressure of $$80 \mathrm{~kPa}$$, then the amount of heat transferred is _______ J.
$$\mathrm{A}_{2}+\mathrm{B}_{2} \rightarrow 2 \mathrm{AB} . \Delta H_{f}^{0}=-200 \mathrm{~kJ} \mathrm{~mol}^{-1}$$
$$\mathrm{AB}, \mathrm{A}_{2}$$ and $$\mathrm{B}_{2}$$ are diatomic molecules. If the bond enthalpies of $$\mathrm{A}_{2}, \mathrm{~B}_{2}$$ and $$\mathrm{AB}$$ are in the ratio $$1: 0.5: 1$$, then the bond enthalpy of $$\mathrm{A}_{2}$$ is ____________ $$\mathrm{kJ} ~\mathrm{mol}^{-1}$$ (Nearest integer)
One mole of an ideal gas at $$350 \mathrm{~K}$$ is in a $$2.0 \mathrm{~L}$$ vessel of thermally conducting walls, which are in contact with the surroundings. It undergoes isothermal reversible expansion from 2.0 L to $$3.0 \mathrm{~L}$$ against a constant pressure of $$4 \mathrm{~atm}$$. The change in entropy of the surroundings ( $$\Delta \mathrm{S})$$ is ___________ $$\mathrm{J} \mathrm{K}^{-1}$$ (Nearest integer)
Given: $$\mathrm{R}=8.314 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}$$.
The total number of intensive properties from the following is __________
Volume, Molar heat capacity, Molarity, $$\mathrm{E}^{\theta}$$ cell, Gibbs free energy change, Molar mass, Mole
Solid fuel used in rocket is a mixture of $$\mathrm{Fe}_{2} \mathrm{O}_{3}$$ and $$\mathrm{Al}$$ (in ratio 1 : 2). The heat evolved $$(\mathrm{kJ})$$ per gram of the mixture is ____________. (Nearest integer)
Given: $$\Delta \mathrm{H}_{\mathrm{f}}^{\theta}\left(\mathrm{Al}_{2} \mathrm{O}_{3}\right)=-1700 \mathrm{~kJ} \mathrm{~mol}^{-1}$$
$$\Delta \mathrm{H}_{\mathrm{f}}^{\theta}\left(\mathrm{Fe}_{2} \mathrm{O}_{3}\right)=-840 \mathrm{~kJ} \mathrm{~mol}^{-1}$$
Molar mass of Fe, Al and O are 56, 27 and 16 g mol$$^{-1}$$ respectively.
The number of endothermic process/es from the following is ______________.
A. $$\mathrm{I}_{2}(\mathrm{~g}) \rightarrow 2 \mathrm{I}(\mathrm{g})$$
B. $$\mathrm{HCl}(\mathrm{g}) \rightarrow \mathrm{H}(\mathrm{g})+\mathrm{Cl}(\mathrm{g})$$
C. $$\mathrm{H}_{2} \mathrm{O}(\mathrm{l}) \rightarrow \mathrm{H}_{2} \mathrm{O}(\mathrm{g})$$
D. $$\mathrm{C}(\mathrm{s})+\mathrm{O}_{2}(\mathrm{~g}) \rightarrow \mathrm{CO}_{2}(\mathrm{~g})$$
E. Dissolution of ammonium chloride in water
For complete combustion of ethene.
$$\mathrm{C}_{2} \mathrm{H}_{4}(\mathrm{g})+3 \mathrm{O}_{2}(\mathrm{g}) \rightarrow 2 \mathrm{CO}_{2}(\mathrm{g})+2 \mathrm{H}_{2} \mathrm{O}(\mathrm{l})$$
the amount of heat produced as measured in bomb calorimeter is $$1406 \mathrm{~kJ} \mathrm{~mol}^{-1}$$ at $$300 \mathrm{~K}$$. The minimum value of $$\mathrm{T} \Delta \mathrm{S}$$ needed to reach equilibrium is ($$-$$) _________ $$\mathrm{kJ}$$. (Nearest integer)
Given : $$\mathrm{R}=8.3 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}$$
When a $$60 \mathrm{~W}$$ electric heater is immersed in a gas for 100 s in a constant volume container with adiabatic walls, the temperature of the gas rises by $$5^{\circ} \mathrm{C}$$. The heat capacity of the given gas is ___________ $$\mathrm{J} \mathrm{K}^{-1}$$ (Nearest integer)
Consider the following data
Heat of combustion of $$\mathrm{H}_{2}(\mathrm{g})\quad\quad=-241.8 \mathrm{~kJ} \mathrm{~mol}^{-1}$$
Heat of combustion of $$\mathrm{C}(\mathrm{s})\quad\quad=-393.5 \mathrm{~kJ} \mathrm{~mol}^{-1}$$
Heat of combustion of $$\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}(\mathrm{l})\quad=-1234.7 \mathrm{~kJ}~{\mathrm{mol}}^{-1}$$
The heat of formation of $$\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}(\mathrm{l})$$ is $$(-)$$ ___________ $$\mathrm{kJ} ~\mathrm{mol}^{-1}$$ (Nearest integer).
Consider the graph of Gibbs free energy G vs Extent of reaction. The number of statement/s from the following which are true with respect to points (a), (b) and (c) is _________

A. Reaction is spontaneous at (a) and (b)
B. Reaction is at equilibrium at point (b) and non-spontaneous at point (c)
C. Reaction is spontaneous at (a) and non-spontaneous at (c)
D. Reaction is non-spontaneous at (a) and (b)
The value of $$\log \mathrm{K}$$ for the reaction $$\mathrm{A} \rightleftharpoons \mathrm{B}$$ at $$298 \mathrm{~K}$$ is ___________. (Nearest integer)
Given: $$\Delta \mathrm{H}^{\circ}=-54.07 \mathrm{~kJ} \mathrm{~mol}^{-1}$$
$$\Delta \mathrm{S}^{\circ}=10 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}$$
(Take $$2.303 \times 8.314 \times 298=5705$$ )
$$0.3 \mathrm{~g}$$ of ethane undergoes combustion at $$27^{\circ} \mathrm{C}$$ in a bomb calorimeter. The temperature of calorimeter system (including the water) is found to rise by $$0.5^{\circ} \mathrm{C}$$. The heat evolved during combustion of ethane at constant pressure is ____________ $$\mathrm{kJ} ~\mathrm{mol}{ }^{-1}$$. (Nearest integer)
[Given : The heat capacity of the calorimeter system is $$20 \mathrm{~kJ} \mathrm{~K}^{-1}, \mathrm{R}=8.3 ~\mathrm{JK}^{-1} \mathrm{~mol}^{-1}$$.
Assume ideal gas behaviour.
Atomic mass of $$\mathrm{C}$$ and $$\mathrm{H}$$ are 12 and $$1 \mathrm{~g} \mathrm{~mol}^{-1}$$ respectively]
At $$25^{\circ} \mathrm{C}$$, the enthalpy of the following processes are given :
| $$\mathrm{H_2(g)+O_2(g)}$$ | $$\to$$ | $$2\mathrm{OH(g)}$$ | $$\mathrm{\Delta H^\circ=78~kJ~mol^{-1}}$$ |
|---|---|---|---|
| $$\mathrm{H_2(g)+\frac{1}{2}O_2(g)}$$ | $$\to$$ | $$\mathrm{H_2O(g)}$$ | $$\mathrm{\Delta H^\circ=-242~kJ~mol^{-1}}$$ |
| $$\mathrm{H_2(g)}$$ | $$\to$$ | $$\mathrm{2H(g)}$$ | $$\mathrm{\Delta H^\circ=436~kJ~mol^{-1}}$$ |
| $$\frac{1}{2}\mathrm{O_2(g)}$$ | $$\to$$ | $$\mathrm{O(g)}$$ | $$\mathrm{\Delta H^\circ=249~kJ~mol^{-1}}$$ |
What would be the value of X for the following reaction ? _____________ (Nearest integer)
$$\mathrm{H_2O(g)\to H(g)+OH(g)~\Delta H^\circ=X~kJ~mol^{-1}}$$
$\mathrm{CCl}_{4}(\mathrm{~g})+2 \mathrm{H}_{2} \mathrm{O}(\mathrm{g}) \rightarrow \mathrm{CO}_{2}(\mathrm{~g})+4 \mathrm{HCl}(\mathrm{g})$
The enthalpy change for the conversion of $$\frac{1}{2} \mathrm{Cl}_{2}(\mathrm{~g})$$ to $$\mathrm{Cl}^{-}$$(aq) is ($$-$$) ___________ $$\mathrm{kJ} \mathrm{mol}^{-1}$$ (Nearest integer)
Given : $$\Delta_{\mathrm{dis}} \mathrm{H}_{\mathrm{Cl}_{2(\mathrm{~g})}^{\theta}}^{\ominus}=240 \mathrm{~kJ} \mathrm{~mol}^{-1}, \Delta_{\mathrm{eg}} \mathrm{H}_{\mathrm{Cl_{(g)}}}^{\ominus}=-350 \mathrm{~kJ} \mathrm{~mol}^{-1}$$,
$${\mathrm{\Delta _{hyd}}H_{Cl_{(g)}^ - }^\Theta = - 380}$$ $$\mathrm{kJ~mol^{-1}}$$
Given $\mathrm{C_V}=20 \mathrm{~J} \mathrm{~mol}^{-1} \mathrm{~K}^{-1}$
When 2 litre of ideal gas expands isothermally into vacuum to a total volume of 6 litre, the change in internal energy is ____________ J. (Nearest integer)
28.0 L of CO$$_2$$ is produced on complete combustion of 16.8 L gaseous mixture of ethene and methane at 25$$^\circ$$C and 1 atm. Heat evolved during the combustion process is ___________ kJ.
Given : $$\mathrm{\Delta H_c~(CH_4)=-900~kJ~mol^{-1}}$$
$$\mathrm{\Delta H_c~(C_2H_4)=-1400~kJ~mol^{-1}}$$
An athlete is given 100 g of glucose (C$$_6$$H$$_{12}$$O$$_6$$) for energy. This is equivalent to 1800kJ of energy. The 50% of this energy gained is utilized by the athlete for sports activities at the event. In order to avoid storage of energy, the weight of extra water he would need to perspire is ____________ g (Nearest integer)
Assume that there is no other way of consuming stored energy.
Given : The enthalpy of evaporation of water is 45 kJ mol$$^{-1}$$
Molar mass of C, H & O are 12, 1 and 16 g mol$$^{-1}$$.
One mole of an ideal monoatomic gas is subjected to changes as shown in the graph. The magnitude of the work done (by the system or on the system) is __________ J (nearest integer)

Given ; $$\log2=0.3$$
$$\ln10=2.3$$
Following figure shows spectrum of an ideal black body at four different temperatures. The number of correct statement/s from the following is ____________.

A. $$\mathrm{T_4 > T_3 > T_2 > T_1}$$
B. The black body consists of particles performing simple harmonic motion.
C. The peak of the spectrum shifts to shorter wavelength as temperature increases.
D. $${{{T_1}} \over {{v_1}}} = {{{T_2}} \over {{v_2}}} = {{{T_3}} \over {{v_3}}} \ne $$ constant
E. The given spectrum could be explained using quantisation of energy.
For independent process at 300 K
| Process | $$\mathrm{\Delta H/kJ~mol^{-1}}$$ | $$\mathrm{\Delta S/J~K^{-1}}$$ |
|---|---|---|
| A | $$-25$$ | $$-80$$ |
| B | $$-22$$ | 40 |
| C | 25 | $$-50$$ |
| D | 22 | 20 |
The number of non-spontaneous process from the following is __________
When 600 mL of 0.2 M HNO3 is mixed with $$400 \mathrm{~mL}$$ of 0.1 M NaOH solution in a flask, the rise in temperature of the flask is ___________ $$\times 10^{-2}{ }\,^{\circ} \mathrm{C}$$.
(Enthalpy of neutralisation $$=57 \mathrm{~kJ} \mathrm{~mol}^{-1}$$ and Specific heat of water $$=4.2 \,\mathrm{JK}^{-1} \mathrm{~g}^{-1}$$) (Neglect heat capacity of flask)
Among the following the number of state variables is ______________.
Internal energy (U)
Volume (V)
Heat (q)
Enthalpy (H)
A gas (Molar mass = 280 $$\mathrm{~g} \mathrm{~mol}^{-1}$$) was burnt in excess $$\mathrm{O}_{2}$$ in a constant volume calorimeter and during combustion the temperature of calorimeter increased from $$298.0 \mathrm{~K}$$ to $$298.45$$ $$\mathrm{K}$$. If the heat capacity of calorimeter is $$2.5 \mathrm{~kJ} \mathrm{~K}^{-1}$$ and enthalpy of combustion of gas is $$9 \mathrm{~kJ} \mathrm{~mol}^{-1}$$ then amount of gas burnt is _____________ g. (Nearest Integer)
The molar heat capacity for an ideal gas at constant pressure is $$20.785 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}$$. The change in internal energy is $$5000 \mathrm{~J}$$ upon heating it from $$300 \mathrm{~K}$$ to $$500 \mathrm{~K}$$. The number of moles of the gas at constant volume is ____________. [Nearest integer] (Given: $$\mathrm{R}=8.314 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}$$)
For the reaction
$$\mathrm{H}_{2} \mathrm{F}_{2}(\mathrm{~g}) \rightarrow \mathrm{H}_{2}(\mathrm{~g})+\mathrm{F}_{2}(\mathrm{~g})$$
$$\Delta U=-59.6 \mathrm{~kJ} \mathrm{~mol}^{-1}$$ at $$27^{\circ} \mathrm{C}$$.
The enthalpy change for the above reaction is ($$-$$) __________ $$\mathrm{kJ} \,\mathrm{mol}^{-1}$$ [nearest integer]
Given: $$\mathrm{R}=8.314 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}$$.
$$2.4 \mathrm{~g}$$ coal is burnt in a bomb calorimeter in excess of oxygen at $$298 \mathrm{~K}$$ and $$1 \mathrm{~atm}$$ pressure. The temperature of the calorimeter rises from $$298 \mathrm{~K}$$ to $$300 \mathrm{~K}$$. The enthalpy change during the combustion of coal is $$-x \mathrm{~kJ} \mathrm{~mol}^{-1}$$. The value of $$x$$ is ___________. (Nearest Integer)
(Given : Heat capacity of bomb calorimeter $$20.0 \mathrm{~kJ} \mathrm{~K}^{-1}$$. Assume coal to be pure carbon)
While performing a thermodynamics experiment, a student made the following observations.
HCl + NaOH $$\to$$ NaCl + H2O $$\Delta$$H = $$-$$57.3 kJ mol$$-$$1
CH3COOH + NaOH $$\to$$ CH3COONa + H2O $$\Delta$$H = $$-$$55.3 kJ mol$$-$$1
The enthalpy of ionization of CH3COOH as calculated by the student is _____________ kJ mol$$-$$1. (nearest integer)
The enthalpy of combustion of propane, graphite and dihydrogen at $$298 \mathrm{~K}$$ are $$-2220.0 \mathrm{~kJ} \mathrm{~mol}^{-1},-393.5 \mathrm{~kJ} \mathrm{~mol}^{-1}$$ and $$-285.8 \mathrm{~kJ} \mathrm{~mol}^{-1}$$ respectively. The magnitude of enthalpy of formation of propane $$\left(\mathrm{C}_{3} \mathrm{H}_{8}\right)$$ is _______________ $$\mathrm{kJ} \,\mathrm{mol}^{-1}$$. (Nearest integer)
1.0 mol of monoatomic ideal gas is expanded from state 1 to state 2 as shown in the figure. The magnitude of the work done for the expansion of gas from state 1 to state 2 at 300 K is ____________ J. (Nearest integer)
(Given : R = 8.3 J K$$-$$1 mol$$-$$1, ln10 = 2.3, log2 = 0.30)

2.2 g of nitrous oxide (N2O) gas is cooled at a constant pressure of 1 atm from 310 K to 270 K causing the compression of the gas from 217.1 mL to 167.75 mL. The change in internal energy of the process, $$\Delta$$U is '$$-$$x' J. The value of 'x' is ________. [nearest integer]
(Given : atomic mass of N = 14 g mol$$-$$1 and of O = 16 g mol$$-$$1. Molar heat capacity of N2O is 100 J K$$-$$1 mol$$-$$1)
17.0 g of NH3 completely vapourises at $$-$$33.42$$^\circ$$C and 1 bar pressure and the enthalpy change in the process is 23.4 kJ mol$$-$$1. The enthalpy change for the vapourisation of 85 g of NH3 under the same conditions is _________ kJ.
For combustion of one mole of magnesium in an open container at 300 K and 1 bar pressure, $$\Delta$$CH$$\Theta $$ = $$-$$601.70 kJ mol$$-$$1, the magnitude of change in internal energy for the reaction is __________ kJ. (Nearest integer)
(Given : R = 8.3 J K$$-$$1 mol$$-$$1)
4.0 L of an ideal gas is allowed to expand isothermally into vacuum until the total volume is 2.0 L. The amount of heat absorbed in this expansion is ____________ L atm.
When 5 moles of He gas expand isothermally and reversibly at 300 K from 10 litre to 20 litre, the magnitude of the maximum work obtained is __________ J. [nearest integer] (Given : R = 8.3 J K$$-$$1 mol$$-$$1 and log 2 = 0.3010)
A fish swimming in water body when taken out from the water body is covered with a film of water of weight 36 g. When it is subjected to cooking at 100$$^\circ$$C, then the internal energy for vaporization in kJ mol$$-$$1 is ___________. [nearest integer]
[Assume steam to be an ideal gas. Given $$\Delta$$vapH$$^\Theta $$ for water at 373 K and 1 bar is 41.1 kJ mol$$-$$1 ; R = 8.31 J K$$-$$1 mol$$-$$1]
For complete combustion of methanol
CH3OH(I) + $${3 \over 2}$$O2(g) $$\to$$ CO2(g) + 2H2O(I)
the amount of heat produced as measured by bomb calorimeter is 726 kJ mol$$-$$1 at 27$$^\circ$$C. The enthalpy of combustion for the reaction is $$-$$x kJ mol$$-$$1, where x is ___________. (Nearest integer)
(Given : R = 8.3 JK$$-$$1 mol$$-$$1)
The standard entropy change for the reaction
4Fe(s) + 3O2(g) $$\to$$ 2Fe2O3(s) is $$-$$550 J K$$-$$1 at 298 K.
[Given : The standard enthalpy change for the reaction is $$-$$165 kJ mol$$-$$1]. The temperature in K at which the reaction attains equilibrium is _____________. (Nearest Integer)
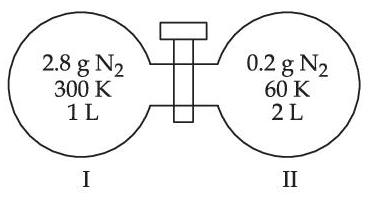
When the valve is opened, the final pressure of the system in bar is x $$\times$$ 10$$-$$2. The value of x is __________. (Integer answer)
[Assume - Ideal gas; 1 bar = 105 Pa; Molar mass of N2 = 28.0 g mol$$-$$1; R = 8.31 J mol$$-$$1 K$$-$$1]
FeO(s) + C(graphite) $$\to$$ Fe(s) + CO(g)
| Substance | $$\Delta H^\circ $$ (kJ mol$$^{ - 1}$$) |
$$\Delta S^\circ $$ (J mol$$^{ - 1}$$ K$$^{ - 1}$$) |
|---|---|---|
| $$Fe{O_{(s)}}$$ | $$ - 266.3$$ | 57.49 |
| $${C_{(graphite)}}$$ | 0 | 5.74 |
| $$F{e_{(s)}}$$ | 0 | 27.28 |
| $$C{O_{(g)}}$$ | $$ - 110.5$$ | 197.6 |
The minimum temperature in K at which the reaction becomes spontaneous is ___________. (Integer answer)
[Given : Specific heat of water = 4.18 J g$$-$$1 K$$-$$1, Density of water = 1.00 g cm$$-$$3]
[Assume no volume change on mixing)
[Use : R = 8.3 J mol$$-$$1 K$$-$$1]
$${\Delta _f}{H^\Theta }$$ for KCl = $$-$$436.7 kJ mol$$-$$1 ;
$${\Delta _{sub}}{H^\Theta }$$ for K = 89.2 kJ mol$$-$$1 ;
$${\Delta _{ionization}}{H^\Theta }$$ for K = 419.0 kJ mol$$-$$1 ;
$${\Delta _{electron\,gain}}{H^\Theta }$$ for Cl(g) = $$-$$348.6 kJ mol$$-$$1 ;
$${\Delta _{bond}}{H^\Theta }$$ for Cl2 = 243.0 kJ mol$$-$$1
The magnitude of lattice enthalpy of KCl in kJ mol$$-$$1 is _____________ (Nearest integer)
[Use : H+ (aq) + OH$$-$$ (aq) $$\to$$ H2O : $$\Delta$$$$\gamma$$H = $$-$$57.1 kJ mol$$-$$1]
Specific heat of H2O = 4.18 J K$$-$$1 g$$-$$1
density of H2O = 1.0 g cm$$-$$3
Assume no change in volume of solution on mixing.
$$\Delta$$vap H $$-$$ $$\Delta$$vap U = _____________ $$\times$$ 102 J mol$$-$$1. (Round off to the Nearest Integer)
[Use : R = 8.31 J mol$$-$$1 K$$-$$1]
[Assume volume of H2O(l) is much smaller than volume of H2O(g). Assume H2O(g) treated as an ideal gas]
the reaction enthalpy $$\Delta$$rH = __________ kJ mol$$-$$1. (Round off to the Nearest Integer).
[ Given : Bond enthalpies in kJ mol$$-$$1 : C-C : 347, C = C : 611; C-H : 414, H-H : 436 ]
For the reaction
3CaO + 2Al $$ \to $$ 3Ca + Al2O3 the standard reaction enthalpy $$\Delta$$rH0 = _________ kJ.
(Round off to the Nearest Integer)
[Given : R = 8.314 J mol$$-$$1 K$$-$$1. Assume, hydrogen is an ideal gas] [Atomic mass of Fe is 55.85 u]
[Given : The values of standard enthalpy of formation of SF6(g), S(g) and F(g) are - 1100, 275 and 80 kJ mol$$-$$1 respectively.]
($${\Delta _r}{H^\Theta }$$ = 80 kJ mol$$-$$1) the entropy change $${\Delta _r}{S^\Theta }$$ depends on the temperature T (in K) as $${\Delta _r}{S^\Theta }$$ = 2T (J K$$-$$1mol$$-$$1).
Minimum temperature at which it will become spontaneous is ___________ K. (Integer)
$$N{H_2}C{H_{(S)}} + {3 \over 2}{O_{2(g)}} \to {N_{2(g)}} + {O_{2(g)}} + {H_2}{O_{(I)}}$$
is _________ kJ. (Rounded off to the nearest integer) [Assume ideal gases and R = 8.314 J mol$$-$$1 K$$-$$1]
$$3HC \equiv C{H_{(g)}} \rightleftharpoons {C_6}{H_{6(l)}}$$
[Given : $${\Delta _f}{G^o}(HC \equiv CH) = - 2.04 \times {10^5}$$ J mol$$-$$1 ; $${\Delta _f}{G^o}({C_6}{H_6}) = - 1.24 \times {10^5}$$ J mol$$-$$1 ; R = 8.314 J K-1 mol$$-$$1]
2A(g) $$ \to $$ A2(g)
at 298 K, $$\Delta $$Uo = –20 kJ mol–1, $$\Delta $$So = –30 JK–1 mol–1,
then the $$\Delta $$Go will be _____ J.
(Given : $$\Delta $$Hvap for water at 373 K = 41 kJ/mol,
R = 8.314 JK–1 mol–1)
A($$l$$) $$ \to $$ 2B(g)
$$\Delta U = 2.1\,kcal,\,\Delta S = 20\,cal\,{K^{ - 1}}$$ at 300 K
Hence $$\Delta $$G in kcal is :
MCQ (Single Correct Answer)
The hydration energies of $K^+$ and $Cl^-$ are $-x$ and $-y$ kJ/mol respectively. If the lattice energy of KCl is $-z$ kJ/mol, then the heat of solution of KCl is :
The correct statement amongst the following is :
Total enthalpy change for freezing of 1 mol of water at $10^{\circ} \mathrm{C}$ to ice at $-10^{\circ} \mathrm{C}$ is ________
(Given: $\Delta_{\text {fus }} \mathrm{H}=x \mathrm{~kJ} / \mathrm{mol}$
$$\begin{aligned} & \mathrm{C}_{\mathrm{p}}\left[\mathrm{H}_2 \mathrm{O}(\mathrm{l})\right]=y \mathrm{~J} \mathrm{~mol}^{-1} \mathrm{~K}^{-1} \\ & \mathrm{C}_{\mathrm{p}}\left[\mathrm{H}_2 \mathrm{O}(\mathrm{~s})\right]=z \mathrm{~J} \mathrm{~mol}^{-1} \mathrm{~K}^{-1} \end{aligned}$$
Consider the given data :
(a) $\mathrm{HCl}(\mathrm{g})+10 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow \mathrm{HCl} .10 \mathrm{H}_2 \mathrm{O} \Delta \mathrm{H}=-69.01 \mathrm{~kJ} \mathrm{~mol}^{-1}$
(b) $\mathrm{HCl}(\mathrm{g})+40 \mathrm{H}_2 \mathrm{O}(\mathrm{l}) \rightarrow \mathrm{HCl} .40 \mathrm{H}_2 \mathrm{O} \Delta \mathrm{H}=-72.79 \mathrm{~kJ} \mathrm{~mol}^{-1}$
Choose the correct statement :
One mole of an ideal gas expands isothermally and reversibly from $10 \mathrm{dm}^3$ to $20 \mathrm{dm}^3$ at 300 K . $\Delta \mathrm{U}, \mathrm{q}$ and work done in the process respectively are
Given: $\mathrm{R}=8.3 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}$
$\ln 10=2.3$
$\log 2=0.30$
$$\log 3=0.48$$
Let us consider a reversible reaction at temperature, T. In this reaction, both $\Delta \mathrm{H}$ and $\Delta \mathrm{S}$ were observed to have positive values. If the equilibrium temperature is Te , then the reaction becomes spontaneous at:
Given below are two statements:
Statement I : When a system containing ice in equilibrium with water (liquid) is heated, heat is absorbed by the system and there is no change in the temperature of the system until whole ice gets melted.
Statement II : At melting point of ice, there is absorption of heat in order to overcome intermolecular forces of attraction within the molecules of water in ice and kinetic energy of molecules is not increased at melting point.
In the light of the above statements, choose the correct answer from the options given below
Arrange the following in order of magnitude of work done by the system/on the system at constant temperature.
(a) $\left|w_{\text {reversible }}\right|$ for expansion in infinite stages.
(b) $\left|w_{\text {irreversible }}\right|$ for expansion in single stage.
(c) $\left|\mathrm{w}_{\text {reversible }}\right|$ for compression in infinite stages.
(d) $\left|w_{\text {irreversible }}\right|$ for compression in single stage.
Choose the correct answer from the options given below :
Which of the following graphs correctly represents the variation of thermodynamic properties of Haber's process?

Two vessels A and B are connected via stopcock. The vessel A is filled with a gas at a certain pressure. The entire assembly is immersed in water and is allowed to come to thermal equilibrium with water. After opening the stopcock the gas from vessel A expands into vessel B and no change in temperature is observed in the thermometer. Which of the following statement is true ?
If $\quad C$ (diamond $) \rightarrow C$ (graphite) $+X \mathrm{~kJ} \mathrm{~mol}^{-1}$
C (diamond) $+\mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{CO}_2(\mathrm{~g})+\mathrm{Y} \mathrm{kJ} \mathrm{mol}{ }^{-1}$
C (graphite) $+\mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{CO}_2(\mathrm{~g})+\mathrm{Z} \mathrm{kJ} \mathrm{mol}^{-1}$
at constant temperature. Then
500 J of energy is transferred as heat to 0.5 mol of Argon gas at 298 K and 1.00 atm. The final temperature and the change in internal energy respectively are: Given: R = 8.3 J K-1 mol-1

An ideal gas undergoes a cyclic transformation starting from the point A and coming back to the same point by tracing the path A→B→C→D→A as shown in the three cases above.
Choose the correct option regarding ΔU :
Ice and water are placed in a closed container at a pressure of 1 atm and temperature 273.15 K . If pressure of the system is increased 2 times, keeping temperature constant, then identify correct observation from following
$$\begin{aligned} & \mathrm{S}(\mathrm{~g})+\frac{3}{2} \mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{SO}_3(\mathrm{~g})+2 x \mathrm{kcal} \\ & \mathrm{SO}_2(\mathrm{~g})+\frac{1}{2} \mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{SO}_3(\mathrm{~g})+y \mathrm{kcal} \end{aligned}$$
The heat of formation of $\mathrm{SO}_2(\mathrm{~g})$ is given by :
Which of the following mixing of 1 M base and 1 M acid leads to the largest increase in temperature?
Let us consider an endothermic reaction which is non-spontaneous at the freezing point of water. However, the reaction is spontaneous at boiling point of water. Choose the correct option.
The effect of temperature on spontaneity of reactions are represented as :
| $\Delta$H | $\Delta$S | Temperature | Spontaneity | |
|---|---|---|---|---|
| (A) | $+$ | $-$ | any T | Non spontaneous |
| (B) | $+$ | $+$ | low T | spontaneous |
| (C) | $-$ | $-$ | low T | Non spontaneous |
| (D) | $-$ | $+$ | any T | spontaneous |
The incorrect combinations are :
Ice at $-5^{\circ} \mathrm{C}$ is heated to become vapor with temperature of $110^{\circ} \mathrm{C}$ at atmospheric pressure. The entropy change associated with this process can be obtained from
Match List - I with List - II.
| List - I (Partial Derivatives) |
List - II (Thermodynamic Quantity) |
||
|---|---|---|---|
| (A) | $\left(\frac{\partial \mathrm{G}}{\partial \mathrm{T}}\right)_{\mathrm{P}}$ | (I) | Cp |
| (B) | $\left(\frac{\partial \mathrm{H}}{\partial \mathrm{T}}\right)_{\mathrm{P}}$ | (II) | $-$S |
| (C) | $\left(\frac{\partial \mathrm{G}}{\partial \mathrm{P}}\right)_{\mathrm{T}}$ | (III) | Cv |
| (D) | $\left(\frac{\partial \mathrm{U}}{\partial \mathrm{T}}\right)_{\mathrm{V}}$ | (IV) | V |
Choose the correct answer from the options given below :
A liquid when kept inside a thermally insulated closed vessel at $25^{\circ} \mathrm{C}$ was mechanically stirred from outside. What will be the correct option for the following thermodynamic parameters ?
Given below are two statements: One is labelled as Assertion (A) and the other is labelled as Reason (R)
Assertion (A) : Enthalpy of neutralisation of strong monobasic acid with strong monoacidic base is always $$-57 \mathrm{~kJ} \mathrm{~mol}^{-1}$$
Reason (R) : Enthalpy of neutralisation is the amount of heat liberated when one mole of $$\mathrm{H}^{+}$$ ions furnished by acid combine with one mole of $${ }^{-} \mathrm{OH}$$ ions furnished by base to form one mole of water.
In the light of the above statements, choose the correct answer from the options given below.
Which of the following is not correct?
What happens when methane undergoes combustion in systems A and B respectively?

Given
| (A) | $$\mathrm{2CO(g)+O_2(g)\to 2CO_2(g)}$$ | $$\mathrm{\Delta H_1^0=-x~kJ~mol^{-1}}$$ | |
|---|---|---|---|
| (B) | $$\mathrm{C(graphite)+O_2(g)\to CO_2(g)}$$ | $$\mathrm{\Delta H_2^0=-y~kJ~mol^{-1}}$$ |
$$\mathrm{\Delta H^0}$$ for the reaction
$$\mathrm{C(graphite)+\frac{1}{2}O_2(g)\to CO(g)}$$ is :
Which of the following relations are correct?
(A) $$\mathrm{\Delta U=q+p\Delta V}$$
(B) $$\mathrm{\Delta G=\Delta H-T\Delta S}$$
(C) $$\Delta \mathrm{S}=\frac{q_{rev}}{T}$$
(D) $$\mathrm{\Delta H=\Delta U-\Delta nRT}$$
Choose the most appropriate answer from the options given below :
Given below are two statements: One is labelled as Assertion $$\mathbf{A}$$ and the other is labelled as Reason $$\mathbf{R}$$
Assertion A : The reduction of a metal oxide is easier if the metal formed is in liquid state than solid state.
Reason $$\mathbf{R}$$ : The value of $$\Delta G ^\Theta$$ becomes more on negative side as entropy is higher in liquid state than solid state.
In the light of the above statements, choose the most appropriate answer from the options given below
Which of the following relation is not correct?
$$\Delta$$G$$^\circ$$ vs T plot for the formation of MgO, involving reaction
2Mg + O2 $$\to$$ 2MgO, will look like :
Match List-I with List-II.
| List - I | List - II | ||
|---|---|---|---|
| (A) | Spontaneous process | (I) | $$\Delta H < 0$$ |
| (B) | Process with $$\Delta P = 0$$, $$\Delta T = 0$$ | (II) | $$\Delta {G_{T,P}} < 0$$ |
| (C) | $$\Delta {H_{reaction}}$$ | (III) | Isothermal and isobaric process |
| (D) | Exothermic Process | (IV) | [Bond energies of molecules in reactants] $$ - $$ [Bond energies of product molecules |
Choose the correct answer from the options given below :
At 25$$^\circ$$C and 1 atm pressure, the enthalpy of combustion of benzene (I) and acetylene (g) are $$-$$ 3268 kJ mol$$-$$1 and $$-$$1300 kJ mol$$-$$1, respectively. The change in enthalpy for the reaction 3 C2H2(g) $$\to$$ C6H6 (I), is :
At 25$$^\circ$$C and 1 atm pressure, the enthalpies of combustion are as given below :
| Substance | $${H_2}$$ | C (graphite) | $${C_2}{H_6}(g)$$ |
|---|---|---|---|
| $${{{\Delta _c}{H^\Theta }} \over {kJ\,mo{l^{ - 1}}}}$$ | $$ - 286.0$$ | $$ - 394.0$$ | $$ - 1560.0$$ |
The enthalpy of formation of ethane is
(A) Freezing of water to ice at 0$$^\circ$$C
(B) Freezing of water to ice at $$-$$10$$^\circ$$C
(C) N2(g) + 3H2(g) $$ \to $$ 2NH3(g)
(D) Adsorption of CO(g) on lead surface.
(E) Dissolution of NaCl in water
Choose the correct answer from the options given below :
4M(s) + nO2(g) $$ \to $$ 2M2On(s)
the free energy change is plotted as a function of temperature. The temperature below which the oxide is stable could be inferred from the plot as the point at which :
The hydration enthalpy of NaCl is :
(a) U and H each depends only on temperature
(b) Compressibility factor z is not equal to 1
(c) CP, m – CV, m = R
(d) dU = CVdT for any process
(A) q + w
(B) q
(C) w
(D) H–TS
(i) C (graphite) + O2(g) $$ \to $$ CO2(g); $$\Delta $$rH$$^\Theta $$ = x kJ mol$$-$$1
(ii) C(graphite) + $${1 \over 2}$$O2(g) $$ \to $$ CO(g); $$\Delta $$rH$$^\Theta $$ = y kJ mol$$-$$1
(iii) CO(g) + $${1 \over 2}$$ O2(g) $$ \to $$ CO2(g); $$\Delta $$rH$$^\Theta $$ = z kJ mol$$-$$1
Based on the above thermochemical equations, find out which one of the following algebraic relationships is correct?
2H2O $$\rightleftharpoons$$ H3O+ + OH$$-$$, the value of $$\Delta $$Go at 298 K is approximately :
Where A and B are non-zero constants. Which of the following is TRUE about this reaction?
The major component of the reaction mixture at T is :
(Specific heat of water liquid and water vapour are 4.2 kJ K$$-$$1 kg$$-$$1 and 2.0 kJ K$$-$$1 kg$$-$$1; heat of liquid fusion and vapourisation of water are 334 kJ$$-$$1 and 2491 kJ kg$$-$$1, respectively). (log 273 = 2.436, log 373 = 2.572, log 383 = 2.583)
(R = 8.314 JK–1 mol–1)
(i) 2Fe2O3(s) $$ \to $$ 4Fe(s) + 3O2(g);
$$\Delta $$rGo = + 1487.0 kJ mol-1
(ii) 2CO(g) + O2(g) $$ \to $$ 2CO2(g);
$$\Delta $$rGo = $$-$$ 514.4 kJ mol-1
Free energy change, $$\Delta $$rGo for the reaction
2Fe2O3(s) + 6CO(g) $$ \to $$ 4Fe(s) + 6CO2(g) will be :
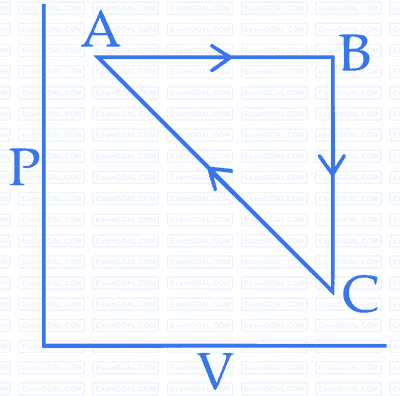
$$\Delta $$UBC = $$-$$5 kJ mol-1, qAB = $$2$$ kJ mol-1, WAB = $$-$$5 kJ mol-1, WCA = 3 kJ mol-1. Heat absorbed by the system during process $$CA$$ is :
(Given $$\Delta $$fusH = 6 kJ mol$$-$$1 at 0oC,
Cp(H2O, $$\ell $$ = 75.3J mol$$-$$1 K$$-$$1)
Cp(H2O s) =36.8 J mol$$-$$1 K$$-$$1)
The correct statement for the reaction is :
$${\Delta _r}{H^o}$$ = - 393.5 kJ mol-1
$${{\rm H}_2}(g)$$ + $${1 \over 2}{O_2}(g)$$$$\to {{\rm H}_2}{\rm O}(l)$$
$${\Delta _r}{H^o}$$ = - 285.8 kJ mol-1
$$C{O_2}(g)$$ + $$2{{\rm H}_2}{\rm O}(l) \to$$ $$C{H_4}(g)$$ + $$2{O_2}(g)$$
$${\Delta _r}{H^o}$$ = + 890.3 kJ mol-1
Based on the above thermochemical equations, the value of $${\Delta _r}{H^o}$$ at 298 K for the reaction
$${C_{(graphite)}}$$ + $$2{{\rm H}_2}(g) \to$$ $$C{H_4}(g)$$ will be :
2H2O2(l) $$\rightleftharpoons$$ 2H2O(l) + O2(g)
(R = 8.3 J K $$-$$1 mol$$-$$1)
A(g) + B(g) $$ \to $$ C(g) + D(g), $$\Delta $$Ho and $$\Delta $$So are, respectively, − 29.8 kJ mol−1 and −0.100 kJ K−1 mol−1 at 298 K. The equilibrium constant for the reaction at 298 K is :
M(s) + $${1 \over 2}$$ O2(g) $$ \to $$ MO(s) and
C(s) + $${1 \over 2}$$ O2(g) $$ \to $$ CO(s)

Identify the correct statement :
2NO(g) + O2 (g) $$\leftrightharpoons$$ 2NO2 (g)
The standard free energy of formation of NO(g) is 86.6 kJ/mol at 298 K. What is the standard free energy of formation of NO2(g) at 298 K? (KP = 1.6 × 1012)
(R = 8.314 J/mol K) ( l n 7.5 = 2.01)
($$\Delta _fG^oH^+_{(aq)}$$ = 0)
H2O(l) $$\to$$ H+(aq) + OH-(aq); $$\Delta H$$ = 57.32 kJ
H2(g) + $${1 \over 2} O_2(g) \to$$ H2O(l); $$\Delta H$$ = -286.20 kJ
The value of enthalpy of formation of OH− ion at 25oC is :
$${1 \over 2}C{l_2}(g)$$ $$\buildrel {{1 \over 2}{\Delta _{diss}}{H^\Theta }} \over \longrightarrow $$ $$Cl(g)$$ $$\buildrel {{\Delta _{eg}}{H^\Theta }} \over \longrightarrow $$ $$C{l^ - }(g)$$ $$\buildrel {{\Delta _{Hyd}}{H^\Theta }} \over \longrightarrow $$ $$C{l^ - }(aq)$$
(Using the data, $${\Delta _{diss}}H_{C{l_2}}^\Theta $$ = 240 kJ/mol, $${\Delta _{eg}}H_{Cl}^\Theta $$ = -349 kJ/mol, $${\Delta _{hyd}}H_{C{l^ - }}^\Theta $$ = - 381 kJ/mol) will be :
$${1 \over 2} X_2$$ + $${3 \over 2} Y_2 \to$$ XY3, $$\Delta H$$ = -30 kJ, to be at equilibrium, the temperature will be :
CaCO3(s) $$\to$$ CaO(s) + CO2 (g) the vales of ∆H° and ∆S° are +179.1 kJ mol−1 and 160.2 J/K respectively at 298 K and 1 bar. Assuming that ∆H° do not change with temperature, temperature above which conversion of limestone to lime will be spontaneous is :
and $$R = 8.3\,J\,mo{l^{ - 1}}\,{K^{ - 1}}$$ )
Cl2(g) = 2Cl(g), 242.3 kJ mol–1
I2(g) = 2I(g), 151.0 kJ mol–1
ICl(g) = I(g) + Cl(g), 211.3 kJ mol–1
I2(s) = I2(g), 62.76 kJ mol–1
Given that the standard states for iodine and chlorine are I2(s) and Cl2(g), the standard enthalpy of formation for ICl(g) is :
H2C = CH2(g) + H2(g) $$\to$$ H3C - CH3(g) at 298 K will be :
2C + O2 $$\to$$ 2CO2; $$\Delta H$$ = -393 J
2Zn + O2 $$\to$$ 2ZnO; $$\Delta H$$ = -412 J