Structure of Atom · Chemistry · JEE Main
MCQ (Single Correct Answer)
Correct statements for an element with atomic number 9 are:
A. There can be 5 electrons for which $m_s = +\frac{1}{2}$ and 4 electrons for which $m_s = -\frac{1}{2}$.
B. There is only one electron in $p_z$ orbital.
C. The last electron goes to orbital with $n = 2$ and $l = 1$.
D. The sum of angular nodes of all the atomic orbitals is 1.
Choose the correct answer from the options given below:
The extra stability of half-filled subshell is due to :
(A) Symmetrical distribution of electrons
(B) Smaller coulombic repulsion energy
(C) The presence of electrons with the same spin in non-degenerate orbitals
(D) Larger exchange energy
(E) Relatively smaller shielding of electrons by one another
Indentify the correct statements :
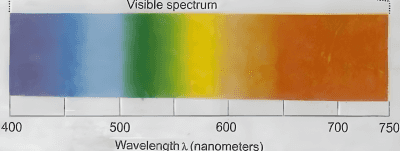
Which of the following statements are correct, if the threshold frequency of caesium is $5.16 \times$ $10^{14} \mathrm{~Hz}$ ?
A. When Cs is placed inside a vacuum chamber with an ammeter connected to it and yellow light is focused on Cs , the ammeter shows the presence of current.
B. When the brightness of the yellow light is dimmed, the value of the current in the ammeter is reduced.
C. When a red light is used instead of the yellow light, the current produced is higher with respect to the yellow light.
D. When a blue light is used, the ammeter shows the formation of current.
E. When a white light is used. the ammeter shows formation of current.
Choose the correct answer from the options given below:
Consider the ground state of chromium atom $(Z=24)$. How many electrons are with Azimuthal quantum number $l=1$ and $l=2$ respectively ?
Which one of the following about an electron occupying the 1 s orbital in a hydrogen atom is incorrect?
(Bohr's radius is represented by $\mathrm{a}_0$)
For electrons in ' 2 s ' and ' 2 p ' orbitals, the orbital angular momentum values, respectively are :
Which of the following statements are true?
(A) The subsidiary quantum number $l$ describes the shape of the orbital occupied by the electron.
(B)  is the boundary surface diagram of the $2 \mathrm{p}_x$ orbital.
is the boundary surface diagram of the $2 \mathrm{p}_x$ orbital.
(C) The + and - signs in the wave function of the $2 p_x$ orbital refer to charge.
(D) The wave function of $2 \mathrm{p}_x$ orbital is zero everywhere in the $x y$ plane.
Choose the correct answer from the options given below :
According to Bohr's model of hydrogen atom, which of the following statement is incorrect?
For hydrogen like species, which of the following graphs provides the most appropriate representation of E vs Z plot for a constant n?
[E : Energy of the stationary state, Z : atomic number, n = principal quantum number]
Given below are two statements :
Statement (I): It is impossible to specify simultaneously with arbitrary precision, both the linear momentum and the position of a particle.
Statement (II) : If the uncertainty in the measurement of position and uncertainty in measurement of momentum are equal for an electron, then the uncertainty in the measurement of velocity is $\geqslant \sqrt{\frac{h}{\pi}} \times \frac{1}{2 m}$.
In the light of the above statements, choose the correct answer from the options given below :
If $a$0 is denoted as the Bohr radius of hydrogen atom, then what is the de-Broglie wavelength (λ) of the electron present in the second orbit of hydrogen atom? [n : any integer]
Which of the following is/are not correct with respect to energy of atomic orbitals of hydrogen atom?
(A) 1s < 2p < 3d < 4s
(B) 1s < 2s = 2p < 3s = 3p
(C) 1s < 2s < 2p < 3s < 3p
(D) 1s < 2s < 4s < 3d
Choose the correct answer from the options given below :
In a multielectron atom, which of the following orbitals described by three quantum numbers will have same energy in absence of electric and magnetic fields?
A. $\mathrm{n}=1, \mathrm{l}=0, \mathrm{~m}_1=0$
B. $\mathrm{n}=2, \mathrm{l}=0, \mathrm{~m}_1=0$
C. $\mathrm{n}=2, \mathrm{I}=1, \mathrm{~m}_1=1$
D. $\mathrm{n}=3, \mathrm{l}=2, \mathrm{~m}_1=1$
E. $\mathrm{n}=3, \mathrm{l}=2, \mathrm{~m}_1=0$
Choose the correct answer from the options given below:
For hydrogen atom, the orbital/s with lowest energy is/are :
(A) $\mathrm{4 s}$
(B) $3 \mathrm{p}_x$
(C) $3 \mathrm{~d}_{x^2-y^2}$
(D) $3 \mathrm{~d}_{z^2}$
(E) $4 \mathrm{p}_z$
Choose the correct answer from the options given below :
Given below are two statements :
Statement (I) : For a given shell, the total number of allowed orbitals is given by $n^2$.
Statement (II) : For any subshell, the spatial orientation of the orbitals is given by $-l$ to $+l$ values including zero.
In the light of the above statements, choose the correct answer from the options given below :
Given below are two statements about X-ray spectra of elements :
Statement (I) : A plot of $\sqrt{v}$ ( $v=$ frequency of X-rays emitted) vs atomic mass is a straight line.
Statement (II) : A plot of $v(\nu=$ frequency of $X$-rays emitted) vs atomic number is a straight line. In the light of the above statements, choose the correct answer from the options given below :
Heat treatment of muscular pain involves radiation of wavelength of about 900 nm . Which spectral line of H atom is suitable for this?
Given : Rydberg constant $\left.\mathrm{R}_{\mathrm{H}}=10^5 \mathrm{~cm}^{-1}, \mathrm{~h}=6.6 \times 10^{-34} \mathrm{~J} \mathrm{~s}, \mathrm{c}=3 \times 10^8 \mathrm{~m} / \mathrm{s}\right)$
Given below are two statements :
Statement (I) : A spectral line will be observed for a $2 p_x \rightarrow 2 p_y$ transition.
Statement (II) : $2 \mathrm{p}_x$ and $2 \mathrm{p}_y$ are degenerate orbitals.
In the light of the above statements, choose the correct answer from the options given below :
Radius of the first excited state of Helium ion is given as : $\mathrm{a}_0 \rightarrow$ radius of first stationary state of hydrogen atom.
The candela is the luminous intensity, in a given direction, of a source that emits monochromatic radiation of frequency '$$A$$' $$\times 10^{12}$$ hertz and that has a radiant intensity in that direction of $$\frac{1}{{ 'B'}}$$, watt per steradian. '$$A$$' and '$$B$$' are respectively
Compare the energies of following sets of quantum numbers for multielectron system.
(A) $$\mathrm{n}=4,1=1$$
(B) $$\mathrm{n}=4,1=2$$
(C) $$\mathrm{n}=3, \mathrm{l}=1$$
(D) $$\mathrm{n}=3,1=2$$
(E) $$\mathrm{n}=4,1=0$$
Choose the correct answer from the options given below :
The incorrect postulates of the Dalton's atomic theory are :
(A) Atoms of different elements differ in mass.
(B) Matter consists of divisible atoms.
(C) Compounds are formed when atoms of different element combine in a fixed ratio.
(D) All the atoms of given element have different properties including mass.
(E) Chemical reactions involve reorganisation of atoms.
Choose the correct answer from the options given below :
Choose the Incorrect Statement about Dalton's Atomic Theory
The four quantum numbers for the electron in the outer most orbital of potassium (atomic no. 19) are
Given below are two statements :
Statement (I) : The orbitals having same energy are called as degenerate orbitals.
Statement (II) : In hydrogen atom, 3p and 3d orbitals are not degenerate orbitals.
In the light of the above statements, choose the most appropriate answer from the options given below :
Match List I with List II
| List - I (Spectral Series for Hydrogen) |
List - II (Spectral Region/Higher Energy State) |
||
|---|---|---|---|
| (A) | Lyman | (I) | Infrared region |
| (B) | Balmer | (II) | UV region |
| (C) | Paschen | (III) | Infrared region |
| (D) | Pfund | (IV) | Visible region |
Choose the correct answer from the options given below:
The correct set of four quantum numbers for the valence electron of rubidium atom $$(\mathrm{Z}=37)$$ is :
Statement I : According to Bohr's model of hydrogen atom, the angular momentum of an electron in a given stationary state is quantised.
Statement II : The concept of electron in Bohr's orbit, violates the Heisenberg uncertainty principle.
In the light of the above statements, choose the most appropriate answer from the options given below:
The energy of an electron in the first Bohr orbit of hydrogen atom is $$-2.18 \times 10^{-18} \mathrm{~J}$$. Its energy in the third Bohr orbit is ____________.
Given below are two statements : one is labelled as Assertion A and the other is labelled as Reason R :
Assertion A : In the photoelectric effect, the electrons are ejected from the metal surface as soon as the beam of light of frequency greater than threshold frequency strikes the surface.
Reason R : When the photon of any energy strikes an electron in the atom, transfer of energy from the photon to the electron takes place.
In the light of the above statements, choose the most appropriate answer from the options given below :
Henry Moseley studied characteristic X-ray spectra of elements. The graph which represents his observation correctly is
Given $$v=$$ frequency of $$\mathrm{X}$$-ray emitted
Z = atomic number
If the radius of the first orbit of hydrogen atom is $$\alpha_{0}$$, then de Broglie's wavelength of electron in $$3^{\text {rd }}$$ orbit is :
Which one of the following sets of ions represents a collection of isoelectronic species?
(Given : Atomic Number : $$\mathrm{F:9,Cl:17,Na=11,Mg=12,Al=13,K=19,Ca=20,Sc=21}$$)
A. $\mathrm{n}=3, \mathrm{l}=0, \mathrm{~m}=0$
B. $\mathrm{n}=4, \mathrm{l}=0, \mathrm{~m}=0$
C. $\mathrm{n}=3, \mathrm{l}=1, \mathrm{~m}=0$
D. $\mathrm{n}=3, \mathrm{l}=2, \mathrm{~m}=1$
The correct option for the order is :
Which transition in the hydrogen spectrum would have the same wavelength as the Balmer type transition from $$\mathrm{n=4}$$ to $$\mathrm{n}=2$$ of $$\mathrm{He}^{+}$$ spectrum
$$ \Psi_{2 \mathrm{~s}}=\frac{1}{2 \sqrt{2 \pi}}\left(\frac{1}{a_0}\right)^{1 / 2}\left(2-\frac{r}{a_0}\right) e^{-r / 2 a_0} $$
At $r=r_0$, radial node is formed. Thus, $r_0$ in terms of $a_0$
The shortest wavelength of hydrogen atom in Lyman series is $$\lambda$$. The longest wavelength is Balmer series of He$$^+$$ is
The radius of the $$\mathrm{2^{nd}}$$ orbit of $$\mathrm{Li^{2+}}$$ is $$x$$. The expected radius of the $$\mathrm{3^{rd}}$$ orbit of $$\mathrm{Be^{3+}}$$ is
The number of s-electrons present in an ion with 55 protons in its unipositive state is
The magnetic moment of a transition metal compound has been calculated to be 3.87 B.M. The metal ion is
It is observed that characteristic X-ray spectra of elements show regularity. When frequency to the power "n" i.e. $${v^n}$$ of X-rays emitted is plotted against atomic number "Z", following graph is obtained.

The value of 'n' is :
Given below are the quantum numbers for 4 electrons.
A. $$\mathrm{n}=3,l=2, \mathrm{~m}_{1}=1, \mathrm{~m}_{\mathrm{s}}=+1 / 2$$
B. $$\mathrm{n}=4,l=1, \mathrm{~m}_{1}=0, \mathrm{~m}_{\mathrm{s}}=+1 / 2$$
C. $$\mathrm{n}=4,l=2, \mathrm{~m}_{1}=-2, \mathrm{~m}_{\mathrm{s}}=-1 / 2$$
D. $$\mathrm{n}=3,l=1, \mathrm{~m}_{1}=-1, \mathrm{~m}_{\mathrm{s}}=+1 / 2$$
The correct order of increasing energy is :
Given below are two statements: One is labelled as Assertion $$\mathbf{A}$$ and the other is labelled as Reason $$\mathbf{R}$$
Assertion $$\mathbf{A}$$ : Zero orbital overlap is an out of phase overlap.
Reason $$\mathbf{R}$$ : It results due to different orientation / direction of approach of orbitals.
In the light of the above statements, choose the correct answer from the options given below
Identify the incorrect statement from the following.
The correct decreasing order of energy for the orbitals having, following set of quantum numbers :
(A) n = 3, l = 0, m = 0
(B) n = 4, l = 0, m = 0
(C) n = 3, l = 1, m = 0
(D) n = 3, l = 2, m = 1
is :
Outermost electronic configurations of four elements A, B, C, D are given below :
(A) $$3 s^{2}$$
(B) $$3 s^{2} 3 p^{1}$$
(C) $$3 s^{2} 3 p^{3}$$
(D) $$3 s^{2} 3 p^{4}$$
The correct order of first ionization enthalpy for them is :
Given below are two statements. One is labelled as Assertion A and the other is labelled as Reason R.
Assertion A: Energy of $$2 \mathrm{s}$$ orbital of hydrogen atom is greater than that of $$2 \mathrm{s}$$ orbital of lithium.
Reason R: Energies of the orbitals in the same subshell decrease with increase in the atomic number.
In the light of the above statements, choose the correct answer from the options given below.
Which of the following sets of quantum numbers is not allowed?
The number of radial nodes and total number of nodes in 4p orbital respectively are :
Which of the following is the correct plot for the probability density $${\psi ^2}$$ (r) as a function of distance 'r' of the electron from the nucleus for 2s orbital?
Which of the following statements are correct?
(A) The electronic configuration of Cr is [Ar] 3d5 4s1.
(B) The magnetic quantum number may have a negative value.
(C) In the ground state of an atom, the orbitals are filled in order of their increasing energies.
(D) The total number of nodes are given by n $$-$$ 2.
Choose the most appropriate answer from the options given below :
Consider the following statements :
(A) The principal quantum number 'n' is a positive integer with values of 'n' = 1, 2, 3, ...
(B) The azimuthal quantum number 'l' for a given 'n' (principal quantum number) can have values as 'l' = 0, 1, 2, ...... n
(C) Magnetic orbital quantum number 'ml' for a particular 'l' (azimuthal quantum number) has (2l + 1) values.
(D) $$\pm$$ 1/2 are the two possible orientations of electron spin.
(E) For l = 5, there will be a total of 9 orbital
Which of the above statements are correct?
The number of radial and angular nodes in 4d orbital are, respectively
If the radius of the 3rd Bohr's orbit of hydrogen atom is r3 and the radius of 4th Bohr's orbit is r4. Then :
The minimum energy that must be possessed by photons in order to produce the photoelectric effect with platinum metal is :
[Given : The threshold frequency of platinum is 1.3 $$\times$$ 1015 s$$-$$1 and h = 6.6 $$\times$$ 10$$-$$34 J s.]
The pair, in which ions are isoelectronic with AI3+ is :
The energy of one mole of photons of radiation of wavelength 300 nm is
(Given : h = 6.63 $$\times$$ 10$$-$$34 J s, NA = 6.02 $$\times$$ 1023 mol$$-$$1, c = 3 $$\times$$ 108 m s$$-$$1)
Consider the following pairs of electrons
(A) (a) n = 3, $$l$$ = 1, m1 = 1, ms = + $${1 \over 2}$$
(b) n = 3, 1 = 2, m1 = 1, ms = + $${1 \over 2}$$
(B) (a) n = 3, $$l$$ = 2, m1 = $$-$$2, ms = $$-$$$${1 \over 2}$$
(b) n = 3, $$l$$ = 2, m1 = $$-$$1, ms = $$-$$$${1 \over 2}$$
(C) (a) n = 4, $$l$$ = 2, m1 = 2, ms = + $${1 \over 2}$$
(b) n = 3, $$l$$ = 2, m1 = 2, ms = + $${1 \over 2}$$
The pairs of electrons present in degenerate orbitals is/are :
Statement I : According to Bohr's model of an atom, qualitatively the magnitude of velocity of electron increases with decrease in positive charges on the nucleus as there is no strong hold on the electron by the nucleus.
Statement II : According to Bohr's model of an atom, qualitatively the magnitude of velocity of electron increases with decrease in principal quantum number.
In the light of the above statements, choose the most appropriate answer from the options given below :
Statement I : Rutherford's gold foil experiment cannot explain the line spectrum of hydrogen atom.
Statement II : Bohr's model of hydrogen atom contradicts Heisenberg' uncertainty principle.
In the light of the above statements, choose the most appropriate answer from the options given below :
Statement I : Bohr's theory accounts for the stability and line spectrum of Li+ ion.
Statement II : Bohr's theory was unable to explain the splitting of spectral lines in the presence of a magnetic field.
In the light of the above statements, choose the most appropriate answer from the options given below :

The correct plot for 3s orbital is :
(A) Kinetic energy of electron is $$ \propto {{{Z^2}} \over {{n^2}}}$$.
(B) The product of velocity (v) of electron and principal quantum number (n), $$'vn' \propto {Z^2}$$.
(C) Frequency of revolution of electron in an orbit is $$ \propto {{{Z^3}} \over {{n^3}}}$$.
(D) Coulombic force of attraction on the electron is $$ \propto {{{Z^3}} \over {{n^4}}}$$.
Choose the most appropriate answer from the options given below :
orbits of Li2+ is R1 . The difference between the
radii of 3rd and 4th orbits of He+ is $$\Delta $$R2 .
Ratio $$\Delta $$R1 : $$\Delta $$R2 is :
$$\overline \nu = {R_H}\left\{ {{1 \over {n_1^2}} - {1 \over {n_2^2}}} \right\}$$, the correct statements among (I) to (IV) are :
(I) As wavelength decreases, the lines in the series converge
(II) The integer n1 is equal to 2
(III) The lines of longest wavelength corresponds to n2 = 3
(IV) The ionization energy of hydrogen can be calculated from wave number of these lines
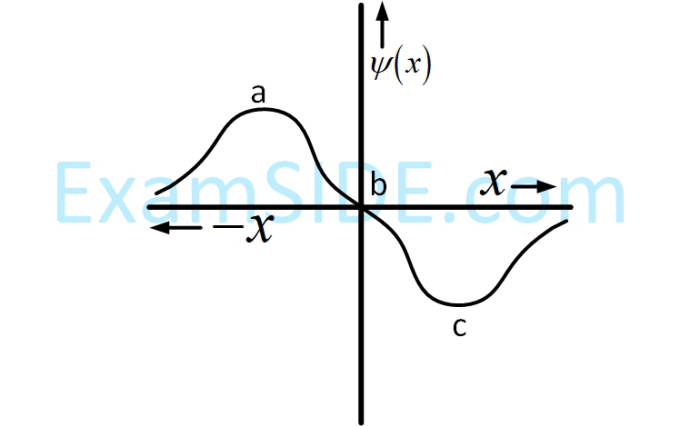

(The Bohr radius is represented by a0)
n = 4, l = 2, ml =–2, ms = –1/2
n = 3, l = 2, ml = 1, ms = +1/2
n = 4, l = 1, ml = 0, ms = +1/2
n = 3, l = 1, ml = 1, ms = –1/2
The correct order of their increasing enegies will be :
(Mass of electron = 9 $$ \times $$ 10–31 kg;
Velocity of light = 3 $$ \times $$ 108 ms$$-$$1
Plank's constant = 6.626 $$ \times $$ 10–34 Js;
Charge of electron = 1.6 $$ \times $$10–19 JeV–1)
[RH = 1 $$ \times $$ 105 cm–1, h = 6.6 $$ \times $$ 10–34 Js, c = 3 $$ \times $$ 108 ms–1]
(a) An electron in an orbital of high angular momentum stays away from the nucleus than an electron in the orbital of lower angular momentum.
(b) For a given value of the principal quantum number, the size of the orbit is inversely proportional to the azimuthal quantum number.
(c) According to wave mechanics, the ground state angular momentum is equal to $${h \over {2\pi }}$$.
(d) The plot of $$\psi $$ vs r for various azimuthal quantum numbers, shows peak shifting towards higher r value.
(Planck’s Const. h = 6.6262 × 10-34 Js; mass of electron = 9.1091 × 10-31 kg; charge of electron (e) = 1.60210 × 10-19 C; permittivity of vacuum ($${\varepsilon _0}$$) = 8.854185 × 10-12 kg-1 m-3 A2)
(h = 6.62 × 10−34 Js and c = 3.0 × 108 ms−1)
(a) n = 4, $$l$$ = 1
(b) n = 4, $$ l$$ = 0
(c) n = 3, $$l$$ = 2
(d) n = 3, $$l$$ = 1
Can be placed in order of increasing energy as :
(c = 3 x 108 ms–1 and NA = 6.02 x 1023 mol–1)
$$\eqalign{ & \left( i \right)\,\,\,\,\,\,C{H_3}^ + \cr & \left( {ii} \right)\,\,\,\,{H_3}{O^ + } \cr & \left( {iii} \right)\,\,\,N{H_3} \cr & \left( {iv} \right)\,\,\,\,C{H_3}^ - \cr} $$
(A) n = 1, l = 0, m = 0
(B) n = 2, l = 0, m = 0
(C) n = 2, l = 1, m = 1
(D) n = 3, l = 2, m = 1
(E) n = 3, l = 2, m = 0
Numerical
The energy of an electron in the first Bohr orbit of the H-atom is -13.6 eV.
The magnitude of energy value of an electron in the first excited state of Be3+ is ________ eV (nearest integer value).
Based on Heisenberg's uncertainty principle, the uncertainty in the velocity of the electron to be found within an atomic nucleus of diameter $$10^{-15} \mathrm{~m}$$ is ________ $$\times 10^9 \mathrm{~ms}^{-1}$$ (nearest integer)
[Given : mass of electron $$=9.1 \times 10^{-31} \mathrm{~kg}$$, Plank's constant $$(h)=6.626 \times 10^{-34} \mathrm{Js}$$] (Value of $$\pi=3.14$$)
Wavenumber for a radiation having 5800 $$\mathop A\limits^o $$ wavelength is $$x \times 10 \mathrm{~cm}^{-1}$$ The value of $$x$$ is ________. (Integer answer)
A hypothetical electromagnetic wave is show below.

The frequency of the wave is $$\mathrm{x} \times 10^{19} \mathrm{~Hz}$$.
$$\mathrm{x}=$$ _________ (nearest integer)
For hydrogen atom, energy of an electron in first excited state is $$-3.4 \mathrm{~eV}, \mathrm{K} . \mathrm{E}$$. of the same electron of hydrogen atom is $$x \mathrm{~eV}$$. Value of $$x$$ is _________ $$\times 10^{-1} \mathrm{~eV}$$. (Nearest integer)
Frequency of the de-Broglie wave of electron in Bohr's first orbit of hydrogen atom is _________ $$\times 10^{13} \mathrm{~Hz}$$ (nearest integer).
[Given : $$\mathrm{R}_{\mathrm{H}}$$ (Rydberg constant) $$=2.18 \times 10^{-18} \mathrm{~J}, h$$ (Plank's constant) $$=6.6 \times 10^{-34} \mathrm{~J} . \mathrm{s}$$.]
In an atom, total number of electrons having quantum numbers $$\mathrm{n}=4,\left|\mathrm{~m}_l\right|=1$$ and $$\mathrm{m}_{\mathrm{s}}=-\frac{1}{2}$$ is _________.
The value of Rydberg constant $$(R_H)$$ is $$2.18 \times 10^{-18} \mathrm{~J}$$. The velocity of electron having mass $$9.1 \times 10^{-31} \mathrm{~kg}$$ in Bohr's first orbit of hydrogen atom = ________ $$\times 10^5 \mathrm{~ms}^{-1}$$ (nearest integer).
The maximum number of orbitals which can be identified with $$\mathrm{n}=4$$ and $$m_l=0$$ is _________.
The de-Broglie's wavelength of an electron in the $$4^{\text {th }}$$ orbit is ________ $$\pi \mathrm{a}_0$$. ($$\mathrm{a}_0=$$ Bohr's radius)
The ionization energy of sodium in $$\mathrm{~kJ} \mathrm{~mol}^{-1}$$, if electromagnetic radiation of wavelength $$242 \mathrm{~nm}$$ is just sufficient to ionize sodium atom is _______.
Number of spectral lines obtained in $$\mathrm{He}^{+}$$ spectra, when an electron makes transition from fifth excited state to first excited state will be
The number of electrons present in all the completely filled subshells having $$\mathrm{n}=4$$ and $$\mathrm{s}=+\frac{1}{2}$$ is _______.
(Where $$\mathrm{n}=$$ principal quantum number and $$\mathrm{s}=$$ spin quantum number)
$\mathrm{O}^{2-}, \mathrm{F}^{-}, \mathrm{Al}, \mathrm{Mg}^{2+}, \mathrm{Na}^{+}, \mathrm{O}^{+}, \mathrm{Mg}, \mathrm{Al}^{3+}, \mathrm{F}$
The orbital angular momentum of an electron in $$3 \mathrm{~s}$$ orbital is $$\frac{x h}{2 \pi}$$. The value of $$x$$ is ____________ (nearest integer)
Values of work function (W$$_0$$) for a few metals are given below
| Metal | Li | Na | K | Mg | Cu | Ag |
|---|---|---|---|---|---|---|
| W$$_0$$/eV | 2.42 | 2.3 | 2.25 | 3.7 | 4.8 | 4.3 |
The number of metals which will show photoelectric effect when light of wavelength $$400 \mathrm{~nm}$$ falls on it is ___________
Given: $$\mathrm{h}=6.6 \times 10^{-34} \mathrm{~J} \mathrm{~s}$$
$$c=3 \times 10^{8} \mathrm{~ms}^{-1}$$
$$e=1.6 \times 10^{-19} \mathrm{C}$$
The number of correct statements from the following is ___________.
A. For $$1 \mathrm{s}$$ orbital, the probability density is maximum at the nucleus
B. For $$2 \mathrm{s}$$ orbital, the probability density first increases to maximum and then decreases sharply to zero.
C. Boundary surface diagrams of the orbitals encloses a region of $$100 \%$$ probability of finding the electron.
D. p and d-orbitals have 1 and 2 angular nodes respectively.
E. probability density of p-orbital is zero at the nucleus

The electron in the $$\mathrm{n}^{\text {th }}$$ orbit of $$\mathrm{Li}^{2+}$$ is excited to $$(\mathrm{n}+1)$$ orbit using the radiation of energy $$1.47 \times 10^{-17} \mathrm{~J}$$ (as shown in the diagram). The value of $$\mathrm{n}$$ is ___________
Given: $$\mathrm{R}_{\mathrm{H}}=2.18 \times 10^{-18} \mathrm{~J}$$
The number of incorrect statement/s about the black body from the following is __________
(A) Emit or absorb energy in the form of electromagnetic radiation.
(B) Frequency distribution of the emitted radiation depends on temperature.
(C) At a given temperature, intensity vs frequency curve passes through a maximum value.
(D) The maximum of the intensity vs frequency curve is at a higher frequency at higher temperature compared to that at lower temperature.
The number of atomic orbitals from the following having 5 radial nodes is ___________.
$$7 \mathrm{s}, 7 \mathrm{p}, 6 \mathrm{s}, 8 \mathrm{p}, 8 \mathrm{d}$$
The number of following statement/s which is/are incorrect is ___________
(A) Line emission spectra are used to study the electronic structure
(B) The emission spectra of atoms in the gas phase show a continuous spread of wavelength from red to violet
(C) An absorption spectrum is like the photographic negative of an emission spectrum
(D) The element helium was discovered in the sun by spectroscopic method
The wavelength of an electron of kinetic energy $$4.50\times10^{-29}$$ J is _________ $$\times 10^{-5}$$ m. (Nearest integer)
Given : mass of electron is $$9\times10^{-31}$$ kg, h $$=6.6\times10^{-34}$$ J s
Electrons in a cathode ray tube have been emitted with a velocity of 1000 m s$$^{-1}$$. The number of following statements which is/are $$\underline {\mathrm{true}} $$ about the emitted radiation is ____________.
Given : $$\mathrm{h=6\times10^{-34}~J~s,m_e=9\times10^{-31}~kg}$$.
(A) The de-Broglie wavelength of the electron emitted is 666.67 nm.
(B) The characteristic of electrons emitted depend upon the material of the electrodes of the cathode ray tube.
(C) The cathode rays start from cathode and move towards anode.
(D) The nature of the emitted electrons depends on the nature of the gas present in cathode ray tube.
The energy of one mole of photons of radiation of frequency $$2 \times 10^{12} \mathrm{~Hz}$$ in $$\mathrm{J} ~\mathrm{mol}^{-1}$$ is ___________. (Nearest integer)
[Given : $$\mathrm{h}=6.626 \times 10^{-34} ~\mathrm{Js}$$
$$\mathrm{N}_{\mathrm{A}}=6.022 \times 10^{23} \mathrm{~mol}^{-1}$$]
Assume that the radius of the first Bohr orbit of hydrogen atom is 0.6 $$\mathrm{\mathop A\limits^o }$$. The radius of the third Bohr orbit of He$$^+$$ is __________ picometer. (Nearest Integer)
The number of given orbitals which have electron density along the axis is _________
$$\mathrm{p_x,p_y,p_z,d_{xy},d_{yz},d_{xz},d_{z^2},d_{x^2-y^2}}$$
If wavelength of the first line of the Paschen series of hydrogen atom is 720 nm, then the wavelength of the second line of this series is _________ nm. (Nearest integer)
The minimum uncertainty in the speed of an electron in an one dimensional region of length $$2 \mathrm{a}_{\mathrm{o}}$$ (Where $$\mathrm{a}_{\mathrm{o}}=$$ Bohr radius $$52.9 \,\mathrm{pm}$$) is _________ $$\mathrm{km} \,\mathrm{s}^{-1}$$.
(Given : Mass of electron = 9.1 $$\times$$ 10$$-$$31 kg, Planck's constant h = 6.63 $$\times$$ 10$$-$$34 Js)
If the wavelength for an electron emitted from $$\mathrm{H}$$-atom is $$3.3 \times 10^{-10} \mathrm{~m}$$, then energy absorbed by the electron in its ground state compared to minimum energy required for its escape from the atom, is _________ times. (Nearest integer)
$$\left[\right.$$ Given $$: \mathrm{h}=6.626 \times 10^{-34} \mathrm{~J} \mathrm{~s}$$ ]
Mass of electron $$=9.1 \times 10^{-31} \mathrm{~kg}$$
Consider an imaginary ion $${ }_{22}^{48} \mathrm{X}^{3-}$$. The nucleus contains '$$a$$'% more neutrons than the number of electrons in the ion. The value of 'a' is _______________. [nearest integer]
The wavelength of an electron and a neutron will become equal when the velocity of the electron is $$x$$ times the velocity of neutron. The value of $$x$$ is ____________. (Nearest Integer)
(Mass of electron is $$9.1 \times 10^{-31} \mathrm{~kg}$$ and mass of neutron is $$1.6 \times 10^{-27} \mathrm{~kg}$$ )
When the excited electron of a H atom from n = 5 drops to the ground state, the maximum number of emission lines observed are _____________.
If the work function of a metal is 6.63 $$\times$$ 10$$-$$19J, the maximum wavelength of the photon required to remove a photoelectron from the metal is ____________ nm. (Nearest integer)
[Given : h = 6.63 $$\times$$ 10$$-$$34 J s, and c = 3 $$\times$$ 108 m s$$-$$1]
Consider the following set of quantum numbers.
| n | 1 | m$$_1$$ | |
|---|---|---|---|
| A. | 3 | 3 | $$ - $$3 |
| B. | 3 | 2 | $$ - $$2 |
| C. | 2 | 1 | +1 |
| D. | 2 | 2 | +2 |
The number of correct sets of quantum numbers is __________.
If the uncertainty in velocity and position of a minute particle in space are, 2.4 $$\times$$ 10$$-$$26 (m s$$-$$1) and 10$$-$$7 (m) respectively. The mass of the particle in g is ____________. (Nearest integer)
(Given : h = 6.626 $$\times$$ 10$$-$$34 Js)
The longest wavelength of light that can be used for the ionisation of lithium atom (Li) in its ground state is x $$\times$$ 10$$-$$8 m. The value of x is ___________. (Nearest Integer).
(Given : Energy of the electron in the first shell of the hydrogen atom is $$-$$2.2 $$\times$$ 10$$-$$18 J ; h = 6.63 $$\times$$ 10$$-$$34 Js and c = 3 $$\times$$ 108 ms$$-$$1)
(h = 6.63 $$\times$$ 10$$-$$34 Js, c = 3.00 $$\times$$ 108 ms$$-$$1)
[Use : h = 6.63 $$\times$$ 10$$-$$34 Js, me = 9.0 $$\times$$ 10$$-$$31 kg]
[Use mass of electron = 9.1 $$\times$$ 10$$-$$31 kg, h =6.63 $$\times$$ 10$$-$$34 Js, $$\pi$$ = 3.14]
(h = 6.626 $$\times$$ 10$$-$$34 Js)
Give : Mass of electron = 9.1 $$\times$$ 10$$-$$31 kg
Charge on an electron = 1.6 $$\times$$ 10$$-$$19 C
Planck's constant = 6.63 $$\times$$ 10$$-$$34 Js
(Atomic number of Ga = 31)
[ Use : $$\sqrt 3 $$ = 1.73, h = 6.63 $$\times$$ 10$$-$$34 Js
me = 9.1 $$\times$$ 10$$-$$31 kg; c = 3.0 $$\times$$ 108 ms$$-$$1; 1eV = 1.6 $$\times$$ 10$$-$$19 J]
[Given : h = 6.63 $$\times$$ 10$$-$$34 Js]
[h = 6.63 $$\times$$ 10$$-$$34 Js, c = 3.00 $$\times$$ 108 ms$$-$$1, NA = 6.02 $$\times$$ 1023 mol$$-$$1]
(A) Li
(B) Na
(C) Rb
(D) Cs
The value of x is ______. (Rounded off to the nearest integer)
[Mass of Li3+ = 8.3 mass of proton]