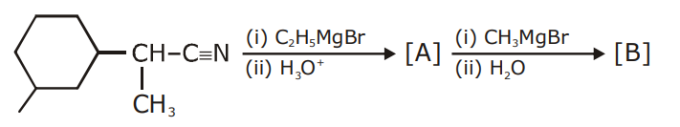Compounds Containing Nitrogen · Chemistry · JEE Main
MCQ (Single Correct Answer)
$\mathrm{A} \xrightarrow[\text { (i) } \mathrm{H}_3 \mathrm{O}^{+}]{\text {(i) } \mathrm{NaH}} \mathrm{B} \xrightarrow[\text { (ii) } \mathrm{H}_2 \mathrm{SO}_4, \Delta]{\text { (i) } \mathrm{EOH}} \mathrm{C}$
' A ' shows positive Lassaign's test for N and its molar mass is 121 .
' B ' gives effervescence with aq $\mathrm{NaHCO}_3$.
'C' gives fruity smell.
Identify $\mathrm{A}, \mathrm{B}$ and C from the following.
Match List - I with List - II.
| List - I (Conversion) | List - II (Reagents, Conditions used) |
|---|---|
 |
(I) Warm H2O |
 |
(II) (a) NaOH, 368K; (b) H3O+ |
 |
(III) (a) NaOH, 443K; (b) H3O+ |
 |
(IV) (a) NaOH, 623K, 300 atm; (b) H3O+ |
Choose the correct answer from the options given below :
Which of the following amine (s) show (s) positive carbylamine test?

B. $\left(\mathrm{CH}_3\right)_2 \mathrm{NH}$
C. $\mathrm{CH}_3 \mathrm{NH}_2$
D. $\left(\mathrm{CH}_3\right)_3 \mathrm{~N}$

Choose the correct answer from the options given below:
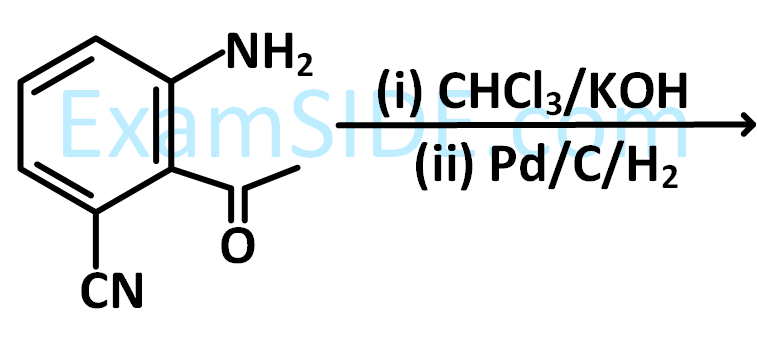
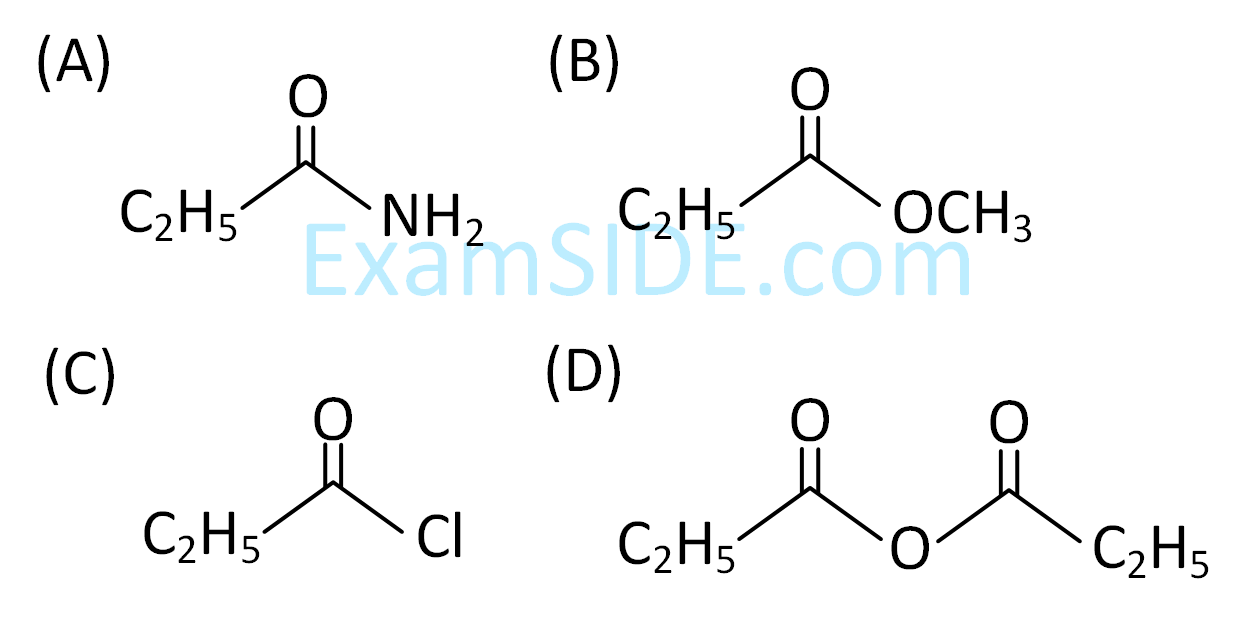
The major product (A) formed in the following reaction sequence is

The sequence from the following that would result in giving predominantly $3,4,5-$ Tribromoaniline is :
$$ \text {Identify }[\mathrm{A}],[\mathrm{B}] \text { and }[\mathrm{C}] \text {, respectively in the following reaction sequence : } $$

$$ \text { In the following reactions, which one is NOT correct? } $$
When a concentrated solution of sulphanilic acid and 1-naphthylamine is treated with nitrous acid $(273 \mathrm{~K})$ and acidified with acetic acid, the mass $(\mathrm{g})$ of 0.1 mole of product formed is :
(Given molar mass in $\mathrm{g} \mathrm{mol}^{-1} \mathrm{H}: 1, \mathrm{C}: 12, \mathrm{~N}: 14, \mathrm{O}: 16, \mathrm{~S}: 32$ )
The correct order of basic nature in aqueous solution for the bases
$\mathrm{NH}_3, \mathrm{H}_2 \mathrm{~N}-\mathrm{NH}_2, \mathrm{CH}_3 \mathrm{CH}_2 \mathrm{NH}_2,\left(\mathrm{CH}_3 \mathrm{CH}_2\right)_2 \mathrm{NH}$ and $\left(\mathrm{CH}_3 \mathrm{CH}_2\right)_3 \mathrm{~N}$ is :
Which one of the following reaction sequences will give an azo dye?
In the following substitution reaction :

Product 'P' formed is :
The steam volatile compounds among the following are :


$\text { Choose the correct answer from the options given below : }$
Identify correct statements :
(A) Primary amines do not give diazonium salts when treated with $\mathrm{NaNO}_2$ in acidic condition.
(B) Aliphatic and aromatic primary amines on heating with $\mathrm{CHCl}_3$ and ethanolic KOH form carbylamines.
(C) Secondary and tertiary amines also give carbylamine test.
(D) Benzenesulfonyl chloride is known as Hinsberg's reagent.
(E) Tertiary amines reacts with benzenesulfonyl chloride very easily.
Choose the correct answer from the options given below :
For reaction

The correct order of set of reagents for the above conversion is :
Given below are two statements I and II.
Statement I : Dumas method is used for estimation of "Nitrogen" in an organic compound.
Statement II : Dumas method involves the formation of ammonium sulphate by heating the organic compound with conc $\mathrm{H}_2 \mathrm{SO}_4$.
In the light of the above statements, choose the correct answer from the options given below
Which among the following react with Hinsberg's reagent?
A. 
B. 
$\text { C. } \mathrm{CH}_3-\mathrm{NH}_2$
D. $\mathrm{N}\left(\mathrm{CH}_3\right)_3$
E. 
Choose the correct answer from the options given below :
Match the Compounds (List - I) with the appropriate Catalyst/ Reagents (List - II) for their reduction into corresponding amines.
| List - I (Compounds) |
List - II (Catalyst/Reagents) |
||
|---|---|---|---|
| (A) |  |
(I) | $$ \mathrm{NaOH} \text { (aqueous) } $$ |
| (B) |  |
(II) | $$ \mathrm{H}_2 / \mathrm{Ni} $$ |
| (C) | $$ \mathrm{R}-\mathrm{C} \equiv \mathrm{~N} $$ |
(III) | $$ \mathrm{LiAlH}_4, \mathrm{H}_2 \mathrm{O} $$ |
| (D) |  |
(IV) | $$ \mathrm{Sn}, \mathrm{HCl} $$ |
Choose the correct answer from the options given below :
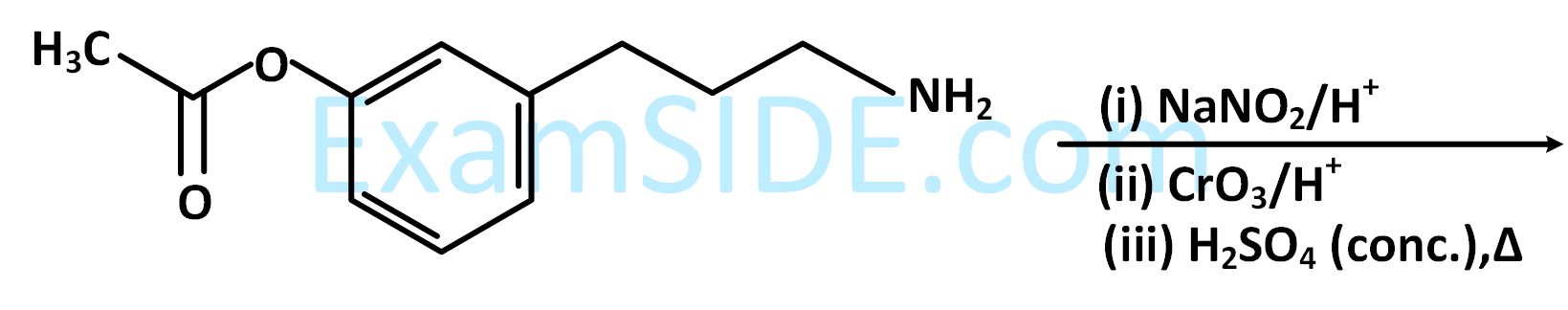
The products formed in the following reaction sequence are :

Major product of the following reaction is

Given below are two statements :
Statement (I) : All the following compounds react with p-toluenesulfonyl chloride.
$$\mathrm{C}_6 \mathrm{H}_5 \mathrm{NH}_2 \quad\left(\mathrm{C}_6 \mathrm{H}_5\right)_2 \mathrm{NH} \quad\left(\mathrm{C}_6 \mathrm{H}_5\right)_3 \mathrm{N}$$
Statement (II) : Their products in the above reaction are soluble is aqueous $$\mathrm{NaOH}$$.
In the light of the above statements, choose the correct answer from the options given below :
Given below are two statements :
Statement (I) : Kjeldahl method is applicable to estimate nitrogen in pyridine.
Statement (II) : The nitrogen present in pyridine can easily be converted into ammonium sulphate in Kjeldahl method.
In the light of the above statements, choose the correct answer from the options given below :
Identify the product A in the following reaction.

Given below are two statements :
Statement I : Piciric acid is 2,4,6 - trinitrotoluene.
Statement II : Phenol - 2,4 - disulphonic acid is treated with Conc. $$\mathrm{HNO}_3$$ to get picric acid.
In the light of the above statements, choose the most appropriate answer from the options given below :
Identify compound (Z) in the following reaction sequence.

Which of the following nitrogen containing compound does not give Lassaigne's test ?
Statement (I) : The $\mathrm{NH}_2$ group in Aniline is ortho and para directing and a powerful activating group.
Statement (II) : Aniline does not undergo Friedel-Craft's reaction (alkylation and acylation).
In the light of the above statements, choose the most appropriate answer from the options given below :
Statement (I) : Aminobenzene and aniline are same organic compounds.
Statement (II) : Aminobenzene and aniline are different organic compounds.
In the light of the above statements, choose the most appropriate answer from the options given below :
The azo-dye $$(Y)$$ formed in the following reactions is
Sulphanilic acid $$+\mathrm{NaNO}_2+\mathrm{CH}_3 \mathrm{COOH} \rightarrow \mathrm{X}$$.

Given below are two statements :
Statement I : Aniline reacts with con. $$\mathrm{H}_2 \mathrm{SO}_4$$, followed by heating at $$453-473 \mathrm{~K}$$ gives $$\mathrm{p}$$-aminobenzene sulphonic acid, which gives blood red colour in the 'Lassaigne's test'.
Statement II : In Friedel - Craft's alkylation and acylation reactions, aniline forms salt with the $$\mathrm{AlCl}_3$$ catalyst. Due to this, nitrogen of aniline aquires a positive charge and acts as deactivating group.
In the light of the above statements, choose the correct answer from the options given below :
The products A and B formed in the following reaction scheme are respectively

Following is a confirmatory test for aromatic primary amines. Identify reagent (A) and (B).

Which of the following reaction is correct?
The product A formed in the following reaction is

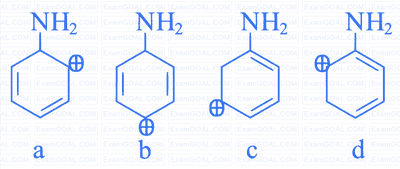
the arenium ion which is not involved in the bromination of Aniline is __________.

The product ' $\mathrm{B}$ ' is

Compound $$\mathrm{A}$$ from the following reaction sequence is:

Match List I with List II
1 - Bromopropane is reacted with reagents in List I to give product in List II
| LIST I - Reagent | LIST II - Product | ||
|---|---|---|---|
| A. | $$\mathrm{KOH}$$ (alc) | I. | Nitrile |
| B. | $$\mathrm{KCN}$$ (alc) | II. | Ester |
| C. | $$\mathrm{AgNO_2}$$ | III. | Alkene |
| D. | $$\mathrm{H_3CCOOAg}$$ | IV. | Nitroalkane |
Choose the correct answer from the options given below:
In the reaction given below

'B' is
The incorrect statement regarding the reaction given below is


'X' is
The major product 'P' formed in the given reaction is:

In the reaction given below:

The product 'X' is:
Isomeric amines with molecular formula C$$_8$$H$$_{11}$$N give the following tests
Isomer (P) $$\Rightarrow$$ Can be prepared by Gabriel phthalimide synthesis
Isomer (Q) $$\Rightarrow$$ Reacts with Hinsberg's reagent to give solid insoluble in NaOH
Isomer (R) $$\Rightarrow$$ Reacts with HONO followed by $$\beta$$-naphthol in NaOH to give red dye.
Isomers (P), (Q) and (R) respectively are
The product ($$\mathrm{P}$$) formed from the following multistep reaction is:

Match List I with List II:

| LIST I (Reagent) | LIST II (Product) | ||
|---|---|---|---|
| A. |  |
I. |  |
| B. | $$\mathrm{HBF_4,\Delta}$$ | II. |  |
| C. | $$\mathrm{Cu,HCl}$$ | III. |  |
| D. | $$\mathrm{CuCN/KCN}$$ | IV. |  |
Choose the correct answer from the options given below:
The major products A and B from the following reactions are:

The major product formed in the following reaction is


Compound $$\mathrm{P}$$ is neutral, $$\mathrm{Q}$$ gives effervescence with $$\mathrm{NaHCO}_{3}$$ while $$\mathrm{R}$$ reacts with Hinsbergs reagent to give solid soluble in $$\mathrm{NaOH}$$. Compound $$\mathrm{P}$$ is
In the following reaction, 'A' is


Consider the above reaction and identify the product B.

In the above conversion of compound $(\mathrm{X})$ to product $(\mathrm{Y})$, the sequence of reagents to be used will be:
Benzyl isocyanide can be obtained by :
A. 
B. 
C. 
D. 
Choose the correct answer from the options given below :
Reaction of propanamide with $$\mathrm{Br_2/KOH(aq)}$$ produces :
The major product 'P' for the following sequence of reactions is :

Match List I with List II
| List I | List II | ||
|---|---|---|---|
| Reaction | Reagents | ||
| (A) | Hoffmann Degradation | (I) | $$\mathrm{Conc. KOH}$$, $$\Delta$$ |
| (B) | Clemenson reduction | (II) | $$\mathrm{CHCl_3,NaOH/H_3O}$$$$^ \oplus $$ |
| (C) | Cannizaro reaction | (III) | $$\mathrm{Br_2,NaOH}$$ |
| (D) | Reimer-Tiemann Reaction | (IV) | $$\mathrm{Zn-Hg/HCl}$$ |
Choose the correct answer from the options given below :
Match List I with List II
| List I (Amines) | List II ($$\mathrm{pK_b}$$) | ||
|---|---|---|---|
| A. | Aniline | I. | 3.25 |
| B. | Ethanamine | II. | 3.00 |
| C. | N-Ethylethanamine | III. | 9.38 |
| D. | N, N-Diethylethanamine | IV. | 3.29 |
Choose the correct answer from the options given below :
Match List I with List II
| List I Isomeric pairs |
List II Type of isomers |
||
|---|---|---|---|
| A. | Propanamine and N-Methylethanamine | I. | Metamers |
| B. | Hexan-2-one and Hexan-3-one | II. | Positional isomers |
| C. | Ethanamide and Hydroxyethanimine | III. | Functional isomers |
| D. | o-nitrophenol and p-nitrophenol | IV. | Tautomers |
Choose the correct answer from the options given below :
Given below are two statements :


In the light of the above statements, choose the correct answer from the options given below:
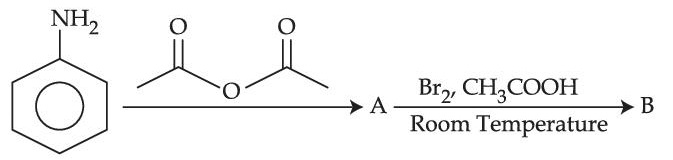
Choose the correct colour of the product for the following reaction.

Given below are two statements:
Statement I : Pure Aniline and other arylamines are usually colourless.
Statement II : Arylamines get coloured on storage due to atmospheric reduction.
In the light of the above statements, choose the most appropriate answer from the options given below :
Increasing order of stability of the resonance structures is :

Choose the correct answer from the options given below :
The Hinsberg reagent is
Which among the following is the strongest Bronsted base?

Consider the above reaction, the compound 'A' is :

Which among the following represent reagent 'A'?
Consider the following reaction sequence:

The product 'B' is :
Given below are two statements: one is labelled as Assertion $$\mathbf{A}$$ and the other is labelled as Reason $$\mathbf{R}$$
Assertion A : Aniline on nitration yields ortho, meta & para nitro derivatives of aniline.
Reason $$\mathrm{R}$$ : Nitrating mixture is a strong acidic mixture.
In the light of the above statements, choose the correct answer from the options given below
Identify the correct statement for the below given transformation.

An organic compound $$'\mathrm{A}'$$ contains nitrogen and chlorine. It dissolves readily in water to give a solution that turns litmus red. Titration of compound $$'\mathrm{A}'$$ with standard base indicates that the molecular weight of $$'\mathrm{A}'$$ is $$131 \pm 2$$. When a sample of $$'\mathrm{A}'$$ is treated with aq. $$\mathrm{NaOH}$$, a liquid separates which contains $$\mathrm{N}$$ but not $$\mathrm{Cl}$$. Treatment of the obtained liquid with nitrous acid followed by phenol gives orange precipitate. The compound $$'\mathrm{A}'$$ is :
Match List I with List II.
| List I | List II | ||
|---|---|---|---|
| (A) | Benzenesulphonyl chloride | (I) | Test for primary amines |
| (B) | Hoffmann bromamide reaction | (II) | Anti Saytzeff |
| (C) | Carbylamine reaction | (III) | Hinsberg reagent |
| (D) | Hoffmann orientation | (IV) | Known reaction of Isocyanates. |
Choose the correct answer from the options given below:
The correct sequential order of the reagents for the given reaction is

The correct stability order of the following diazonium salt is

Given below are two statements : one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A) : Experimental reaction of $$\mathrm{CH}_{3} \mathrm{Cl}$$ with aniline and anhydrous $$\mathrm{AlCl}_{3}$$ does not give $$o$$ and $$p$$-methylaniline.
Reason (R): The $$-\mathrm{NH}_{2}$$ group of aniline becomes deactivating because of salt formation with anhydrous $$\mathrm{AlCl}_{3}$$ and hence yields $$m$$-methyl aniline as the product.
In the light of the above statements, choose the most appropriate answer from the options given below :
An organic compound 'A' on reaction with NH3 followed by heating gives compound B. Which on further strong heating gives compound C (C8H5NO2). Compound C on sequential reaction with ethanolic KOH, alkyl chloride and hydrolysis with alkali gives a primary amine. The compound A is :
In Friedel-Crafts alkylation of aniline, one gets


Consider the above reactions, the product A and product B respectively are
With respect to the following reaction, consider the given statements :

(A) o-Nitroaniline and p-nitroaniline are the predominant products.
(B) p-Nitroaniline and m-nitroaniline are the predominant products.
(C) HNO3 acts as an acid.
(D) H2SO4 acts as an acid.
Choose the correct option.
Identify the major product formed in the following sequence of reactions:

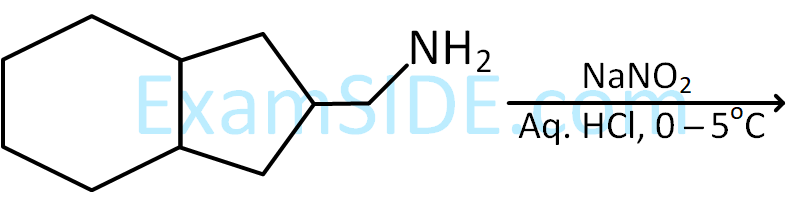
A primary aliphatic amine on reaction with nitrous acid in cold (273 K) and there after raising temperature of reaction mixture to room temperature (298 K), gives a/an
Decarboxylation of all six possible forms of diaminobenzoic acids C6H3(NH2)2COOH yields three products A, B and C. Three acids give a product 'A', two acids gives a product 'B' and one acid give a product 'C'. The melting point of product 'C' is
Given below are two statements :
Statement I : In Hofmann degradation reaction, the migration of only an alkyl group takes place from carbonyl carbon of the amide to the nitrogen atom.
Statement II : The group is migrated in Hofmann degradation reaction to electron deficient atom.
In the light of the above statements, choose the most appropriate answer from the options given below :
Which statement is NOT correct for p-toluenesulphonyl chloride?
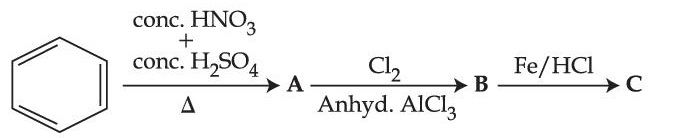
The final product 'C' in the following series of reactions

Among the following structures, which will show the most stable enamine formation? (Where Me is $$-$$CH3)
Amongst the following, the major product of the given chemical reaction is

Which of the following ketone will NOT give enamine on treatment with secondary amines? [where t-Bu is $$-$$C(CH3)3]
The reaction of  with bromine and KOH gives RNH2 as the end product. Which one of the following is the intermediate product formed in this reation?
with bromine and KOH gives RNH2 as the end product. Which one of the following is the intermediate product formed in this reation?
The conversion of propan-1-ol to n-butylamine involves the sequential addition of reagents. The correct sequential order of reagents is

The major product of the above reactions is :


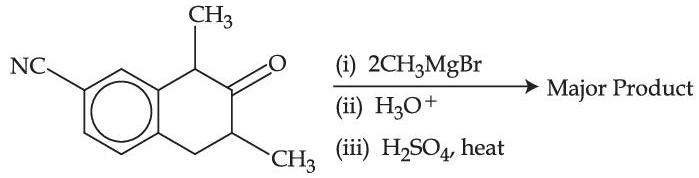


Choose the most appropriate match :

The major product in the above reaction is :

Consider the given reaction, identify 'X' and 'Y' :

Consider the above reaction and identify "Y"

Statement I : Aniline is less basic than acetamide.
Statement II : In aniline, the lone pair of electrons on nitrogen atom is delocalized over benzene ring due to resonance and hence less available to a proton.
Choose the most appropriate option;

Consider the above reaction, the product "P" is :

Assertion (A) : Gabriel phthalimide synthesis cannot be used to prepare aromatic primary amines.
Reason (R) : Aryl halides do not undergo nucleophilic substitution reaction.
In the light of the above statements, choose the correct answer from the options given below :

In the chemical reactions given above A and B respectively are :

In the above reactions, product A and product B respectively are :

Consider the above reaction, compound B is :

Consider the given reaction, percentage yield of :

Considering the above reaction, X and Y respectively are :
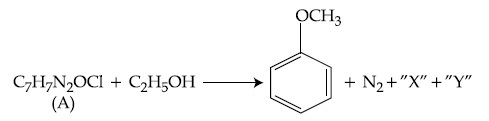
In the above reaction, the structural formula of (A), ''X'' and ''Y'' respectively are :
The structures of A and B are :
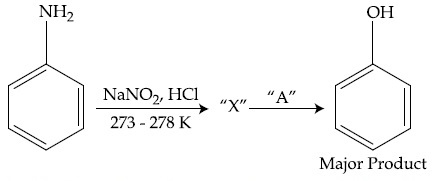
In the above chemical reaction, intermediate ''X'' and reagent/condition ''A'' are :
B. N,N-Dimethylaniline
C. N-Methyl aniline
D. Benzenamine
Choose the correct order of basic nature of the above amines.

Correct statement about the given chemical reaction is :





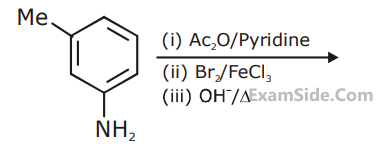


R has lower boiling point than S

A, B and C, respectively are :

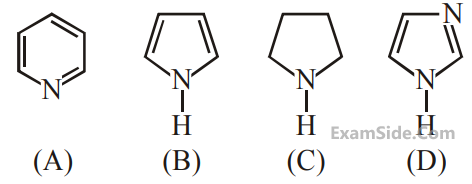
The compound [P] is :
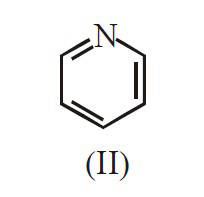
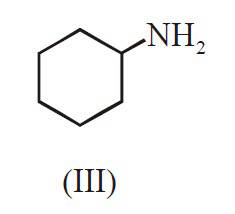
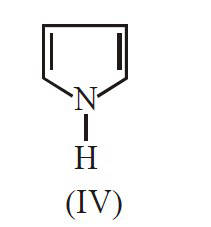



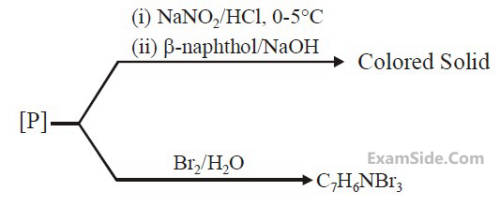
The major product B is :

The product 'X' is used :


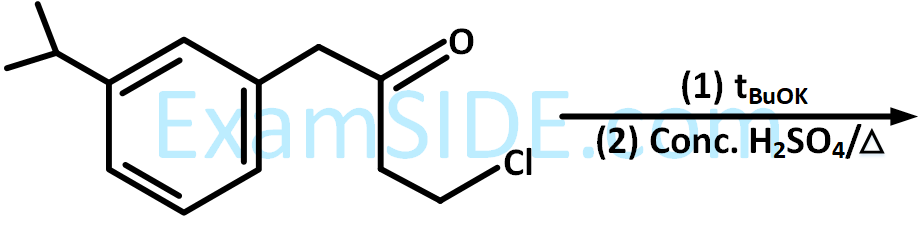

(A)

(B)

(C)

(D)














The product $$E$$ is :
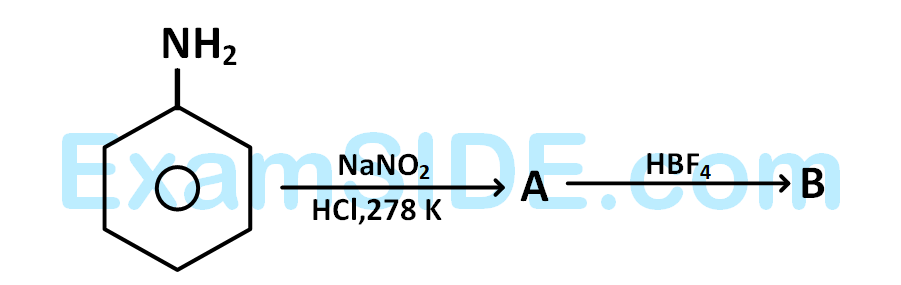
the compounds $$'A'$$ and $$'B'$$ respectively are
CH3CH2NH2 + CHCl3 + 3KOH $$\to$$ (A) + (B) + 3H2O, the compound (A) and (B) are respectively
Numerical
Given below are some nitrogen containing compounds :

Each of them is treated with HCl separately. 1.0 g of the most basic compound will consume _______ mg of HCl.
(Given molar mass in g mol−1 C : 12, H : 1, O : 16, Cl : 35.5)
Consider the following sequence of reactions.

Total number of $\mathrm{sp}^3$ hybridised carbon atoms in the major product C formed is _________.
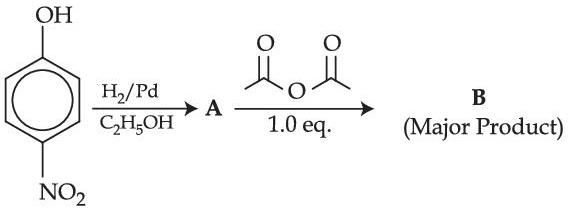
Consider the following sequence of reactions to produce major product (A)

$\begin{aligned} & \text { Molar mass of product }(\mathrm{A}) \text { is } \mathrm{g} \mathrm{~mol}^{-1} \text {. } \\ & \text { (Given molar mass in } \mathrm{g} \mathrm{~mol}^{-1} \text { of } \mathrm{C}: 12, \mathrm{H}: 1, \mathrm{O}: 16, \mathrm{Br}: 80, \mathrm{~N}: 14, \mathrm{P}: 31 \text { ) }\end{aligned}$
Consider the following sequence of reactions :

Molar mass of the product formed $(\mathrm{A})$ is __________ $\mathrm{g} \mathrm{~mol}^{-1}$.
Number of compounds from the following which cannot undergo Friedel-Crafts reactions is: _________
toluene, nitrobenzene, xylene, cumene, aniline, chlorobenzene, $$m$$-nitroaniline, $$m$$-dinitrobenzene
If $$279 \mathrm{~g}$$ of aniline is reacted with one equivalent of benzenediazonium chloride, the maximum amount of aniline yellow formed will be ________ g. (nearest integer)
(consider complete conversion).
Number of amine compounds from the following giving solids which are soluble in $$\mathrm{NaOH}$$ upon reaction with Hinsberg's reagent is _________.

An amine $$(\mathrm{X})$$ is prepared by ammonolysis of benzyl chloride. On adding p-toluenesulphonyl chloride to it the solution remains clear. Molar mass of the amine $$(\mathrm{X})$$ formed is _________ $$\mathrm{g} \mathrm{mol}^{-1}$$.
(Given molar mass in $$\mathrm{gmol}^{-1} \mathrm{C}: 12, \mathrm{H}: 1, \mathrm{O}: 16, \mathrm{~N}: 14$$)
$$9.3 \mathrm{~g}$$ of pure aniline upon diazotisation followed by coupling with phenol gives an orange dye. The mass of orange dye produced (assume 100% yield/conversion) is ________ g. (nearest integer)
$$\mathrm{X} \mathrm{~g}$$ of ethanamine was subjected to reaction with $$\mathrm{NaNO}_2 / \mathrm{HCl}$$ followed by hydrolysis to liberate $$\mathrm{N}_2$$ and $$\mathrm{HCl}$$. The $$\mathrm{HCl}$$ generated was completely neutralised by 0.2 moles of $$\mathrm{NaOH} . \mathrm{X}$$ is _________ g.
$$9.3 \mathrm{~g}$$ of pure aniline is treated with bromine water at room temperature to give a white precipitate of the product '$$\mathrm{P}$$'. The mass of product '$$\mathrm{P}$$' obtained is $$26.4 \mathrm{~g}$$. The percentage yield is ________ %.
Phthalimide is made to undergo following sequence of reactions.

Total number of $$\pi$$ bonds present in product 'P' is/are ________.
The number of the correct reaction(s) among the following is _______.


A compound $$(x)$$ with molar mass $$108 \mathrm{~g} \mathrm{~mol}^{-1}$$ undergoes acetylation to give product with molar mass $$192 \mathrm{~g} \mathrm{~mol}^{-1}$$. The number of amino groups in the compound $$(x)$$ is ___________.
The compound formed by the reaction of ethanal with semicarbazide contains _________ number of nitrogen atoms.
Number of isomeric aromatic amines with molecular formula $$\mathrm{C}_{8} \mathrm{H}_{11} \mathrm{~N}$$, which can be synthesized by Gabriel Phthalimide synthesis is ____________.
How many of the transformations given below would result in aromatic amines?

The number of sp3 hybridised carbons in an acyclic neutral compound with molecular formula C4H5N is ___________.
I. Sn $$-$$ HCl
II. Sn $$-$$ NH4OH
III. Fe $$-$$ HCl
IV. Zn $$-$$ HCl
V. H2 $$-$$ Pd
VI. H2 $$-$$ Raney Nickel