JEE Main 2020 (Online) 3rd September Evening Slot
Paper was held on
Thu, Sep 3, 2020 9:30 AM
Chemistry
1
The d-electron configuration of [Ru(en)3
]Cl2
and [Fe(H2O)6]Cl2 , respectively are :
and [Fe(H2O)6]Cl2 , respectively are :
2
Consider the following reaction :

The product 'P' gives positive ceric ammonium nitrate test. This is because of the presence of which of these –OH group(s)?

The product 'P' gives positive ceric ammonium nitrate test. This is because of the presence of which of these –OH group(s)?
3
For the reaction
2A + 3B + $${3 \over 2}$$C $$ \to $$ 3P, which statement is correct ?
2A + 3B + $${3 \over 2}$$C $$ \to $$ 3P, which statement is correct ?
4
Complex A has a composition of H12O6Cl3Cr. If the complex on treatment with conc.H2SO4
loses
13.5% of its original mass, the correct molecular formula of A is :
[Given: atomic mass of Cr = 52 amu and Cl = 35 amu]
[Given: atomic mass of Cr = 52 amu and Cl = 35 amu]
5
Consider the following molecules and
statements related to them :

(a) (B) is more likely to be crystalline than (A)
(b) (B) has higher boiling point than (A)
(c) (B) dissolves more readily than (A) in water
Identify the correct option from below :

(a) (B) is more likely to be crystalline than (A)
(b) (B) has higher boiling point than (A)
(c) (B) dissolves more readily than (A) in water
Identify the correct option from below :
6
The compound A in the following reaction is :


7
The incorrect statement is :
8
The increasing order of the reactivity of the
following compound in nucleophilic addition
reaction is :
Propanal, Benzaldehyde, Propanone, Butanone
Propanal, Benzaldehyde, Propanone, Butanone
9
The decreasing order of reactivity of the
following compounds towards nucleophilic
substitution (SN2) is :


10
The major product in the following reaction is :


11
100 mL of 0.1 M HCl is taken in a beaker and
to it 100 mL of 0.1 M NaOH is added in steps
of 2 mL and the pH is continuously measured.
Which of the following graphs correctly depicts
the change in pH?
12
Three isomers A, B and C (mol. formula C8H11N) give the following results :

R has lower boiling point than S

A, B and C, respectively are :

R has lower boiling point than S

A, B and C, respectively are :
13
The five successive ionization enthalpies of an
element are 800, 2427, 3658, 25024 and 32824
kJ mol–1. The number of valence electrons in
the element is :
14
Consider the hypothetical situation where the
azimuthal quantum number,
$$l$$, takes values 0,
1, 2, ....., n + 1, where n is the principal
quantum number. Then, the element with
atomic number :
15
Among the statements (I – IV), the correct ones
are:
(I) Be has smaller atomic radius compared to Mg.
(II) Be has higher ionization enthalpy than Al.
(III) Charge/radius ratio of Be is greater than that of Al.
(IV) Both Be and Al form mainly covalent compounds.
(I) Be has smaller atomic radius compared to Mg.
(II) Be has higher ionization enthalpy than Al.
(III) Charge/radius ratio of Be is greater than that of Al.
(IV) Both Be and Al form mainly covalent compounds.
16
The strengths of 5.6 volume hydrogen peroxide
(of density 1 g/mL) in terms of mass percentage
and molarity (M), respectively, are:
(Take molar mass of hydrogen peroxide as
34 g/mol)
17
The number of  groups present in a tripeptide Asp–Glu–Lys is ____.
groups present in a tripeptide Asp–Glu–Lys is ____.
 groups present in a tripeptide Asp–Glu–Lys is ____.
groups present in a tripeptide Asp–Glu–Lys is ____.18
6.023 $$ \times $$ 1022 molecules are present in 10 g of a substance 'x'. The molarity of a solution containing
5 g of substance 'x' in 2 L solution is _____ × 10-3
19
If 250 cm3
of an aqueous solution containing 0.73 g of a protein A is isotonic with one litre of
another aqueous solution containing 1.65 g of a protein B, at 298 K, the ratio of the molecular
masses of A and B is ______ × 10–2 (to the nearest integer).
20
The volume (in mL) of 0.1 N NaOH required to neutralise 10 mL of 0.1 N phosphinic acid is ___________.
21
An acidic solution of dichromate is electrolyzed
for 8 minutes using 2A current. As per the
following equation
Cr2O72- + 14H+ + 6e– $$ \to $$ 2Cr3+ + 7H2O
The amount of Cr3+ obtained was 0.104 g. The efficiency of the process(in%) is (Take : F = 96000 C, At. mass of chromium = 52) ______.
Cr2O72- + 14H+ + 6e– $$ \to $$ 2Cr3+ + 7H2O
The amount of Cr3+ obtained was 0.104 g. The efficiency of the process(in%) is (Take : F = 96000 C, At. mass of chromium = 52) ______.
Mathematics
1
Suppose f(x) is a polynomial of degree four,
having critical points at –1, 0, 1. If
T = {x $$ \in $$ R | f(x) = f(0)}, then the sum of squares of all the elements of T is :
T = {x $$ \in $$ R | f(x) = f(0)}, then the sum of squares of all the elements of T is :
2
Let a, b c $$ \in $$ R be such that a2
+ b2
+ c2
= 1. If
$$a\cos \theta = b\cos \left( {\theta + {{2\pi } \over 3}} \right) = c\cos \left( {\theta + {{4\pi } \over 3}} \right)$$,
where $${\theta = {\pi \over 9}}$$, then the angle between the vectors $$a\widehat i + b\widehat j + c\widehat k$$ and $$b\widehat i + c\widehat j + a\widehat k$$ is :
$$a\cos \theta = b\cos \left( {\theta + {{2\pi } \over 3}} \right) = c\cos \left( {\theta + {{4\pi } \over 3}} \right)$$,
where $${\theta = {\pi \over 9}}$$, then the angle between the vectors $$a\widehat i + b\widehat j + c\widehat k$$ and $$b\widehat i + c\widehat j + a\widehat k$$ is :
3
Let the latus ractum of the parabola y2
= 4x be
the common chord to the circles C1
and C2
each of them having radius 2$$\sqrt 5 $$. Then, the
distance between the centres of the circles C1
and C2
is :
4
Let R1
and R2
be two relation defined as
follows :
R1 = {(a, b) $$ \in $$ R2 : a2 + b2 $$ \in $$ Q} and
R2 = {(a, b) $$ \in $$ R2 : a2 + b2 $$ \notin $$ Q},
where Q is the set of all rational numbers. Then :
R1 = {(a, b) $$ \in $$ R2 : a2 + b2 $$ \in $$ Q} and
R2 = {(a, b) $$ \in $$ R2 : a2 + b2 $$ \notin $$ Q},
where Q is the set of all rational numbers. Then :
5
If the value of the integral
$$\int\limits_0^{{1 \over 2}} {{{{x^2}} \over {{{\left( {1 - {x^2}} \right)}^{{3 \over 2}}}}}} dx$$
is $${k \over 6}$$, then k is equal to :
$$\int\limits_0^{{1 \over 2}} {{{{x^2}} \over {{{\left( {1 - {x^2}} \right)}^{{3 \over 2}}}}}} dx$$
is $${k \over 6}$$, then k is equal to :
6
Let e1
and e2
be the eccentricities of the
ellipse,
$${{{x^2}} \over {25}} + {{{y^2}} \over {{b^2}}} = 1$$(b < 5) and the hyperbola,
$${{{x^2}} \over {16}} - {{{y^2}} \over {{b^2}}} = 1$$ respectively satisfying e1e2 = 1. If $$\alpha $$
and $$\beta $$ are the distances between the foci of the
ellipse and the foci of the hyperbola
respectively, then the ordered pair ($$\alpha $$, $$\beta $$) is equal to :
$${{{x^2}} \over {25}} + {{{y^2}} \over {{b^2}}} = 1$$(b < 5) and the hyperbola,
$${{{x^2}} \over {16}} - {{{y^2}} \over {{b^2}}} = 1$$ respectively satisfying e1e2 = 1. If $$\alpha $$
and $$\beta $$ are the distances between the foci of the
ellipse and the foci of the hyperbola
respectively, then the ordered pair ($$\alpha $$, $$\beta $$) is equal to :
7
Let S be the set of all integer solutions, (x, y, z),
of the system of equations
x – 2y + 5z = 0
–2x + 4y + z = 0
–7x + 14y + 9z = 0
such that 15 $$ \le $$ x2 + y2 + z2 $$ \le $$ 150. Then, the number of elements in the set S is equal to ______ .
x – 2y + 5z = 0
–2x + 4y + z = 0
–7x + 14y + 9z = 0
such that 15 $$ \le $$ x2 + y2 + z2 $$ \le $$ 150. Then, the number of elements in the set S is equal to ______ .
8
If m arithmetic means (A.Ms) and three
geometric means (G.Ms) are inserted between
3 and 243 such that 4th A.M. is equal to 2nd
G.M., then m is equal to _________ .
9
The total number of 3-digit numbers, whose
sum of digits is 10, is __________.
10
The probability that a randomly chosen 5-digit
number is made from exactly two digits is :
11
If x3dy + xy dx = x2dy + 2y dx; y(2) = e and
x > 1, then y(4) is equal to :
x > 1, then y(4) is equal to :
12
$$\mathop {\lim }\limits_{x \to a} {{{{\left( {a + 2x} \right)}^{{1 \over 3}}} - {{\left( {3x} \right)}^{{1 \over 3}}}} \over {{{\left( {3a + x} \right)}^{{1 \over 3}}} - {{\left( {4x} \right)}^{{1 \over 3}}}}}$$ ($$a$$ $$ \ne $$ 0) is equal to :
13
If the surface area of a cube is increasing at a
rate of 3.6 cm2/sec, retaining its shape; then
the rate of change of its volume (in cm3/sec),
when the length of a side of the cube is
10 cm, is :
14
If a $$\Delta $$ABC has vertices A(–1, 7), B(–7, 1) and
C(5, –5), then its orthocentre has coordinates :
15
Let xi
(1 $$ \le $$ i $$ \le $$ 10) be ten observations of a
random variable X. If
$$\sum\limits_{i = 1}^{10} {\left( {{x_i} - p} \right)} = 3$$ and $$\sum\limits_{i = 1}^{10} {{{\left( {{x_i} - p} \right)}^2}} = 9$$
where 0 $$ \ne $$ p $$ \in $$ R, then the standard deviation of these observations is :
$$\sum\limits_{i = 1}^{10} {\left( {{x_i} - p} \right)} = 3$$ and $$\sum\limits_{i = 1}^{10} {{{\left( {{x_i} - p} \right)}^2}} = 9$$
where 0 $$ \ne $$ p $$ \in $$ R, then the standard deviation of these observations is :
16
If z1
, z2
are complex numbers such that
Re(z1) = |z1 – 1|, Re(z2) = |z2 – 1| , and
arg(z1 - z2) = $${\pi \over 6}$$, then Im(z1 + z2 ) is equal to :
Re(z1) = |z1 – 1|, Re(z2) = |z2 – 1| , and
arg(z1 - z2) = $${\pi \over 6}$$, then Im(z1 + z2 ) is equal to :
17
If $$\int {{{\sin }^{ - 1}}\left( {\sqrt {{x \over {1 + x}}} } \right)} dx$$ = A(x)$${\tan ^{ - 1}}\left( {\sqrt x } \right)$$ + B(x) + C,
where C is a constant of integration, then the ordered pair (A(x), B(x)) can be :
where C is a constant of integration, then the ordered pair (A(x), B(x)) can be :
18
If the term independent of x in the expansion of
$${\left( {{3 \over 2}{x^2} - {1 \over {3x}}} \right)^9}$$ is k, then 18 k is equal to :
$${\left( {{3 \over 2}{x^2} - {1 \over {3x}}} \right)^9}$$ is k, then 18 k is equal to :
19
The set of all real values of $$\lambda $$ for which the
quadratic equations,
($$\lambda $$2 + 1)x2 – 4$$\lambda $$x + 2 = 0 always have exactly one root in the interval (0, 1) is :
($$\lambda $$2 + 1)x2 – 4$$\lambda $$x + 2 = 0 always have exactly one root in the interval (0, 1) is :
Physics
1
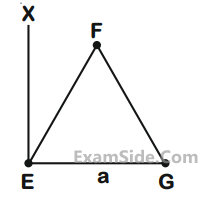
An massless equilateral triangle EFG of side ‘a’ (As shown in figure) has three particles of mass m
situated at its vertices. The moment of inertia of the system about the line EX perpendicular to EG
in the plane of EFG is $${N \over {20}}$$ ma2
where N is an integer. The value of N is _____.

2
A galvanometer coil has 500 turns and each turn has an average area of 3 $$ \times $$ 10–4 m2
. If a torque of
1.5 Nm is required to keep this coil parallel to a magnetic field when a current of 0.5 A is flowing
through it, the strength of the field (in T) is ______.
3
A block starts moving up an inclined plane of inclination 30o with an initial velocity of v0
. It comes
back to its initial position with velocity $${{{v_0}} \over 2}$$. The value of the coefficient of kinetic friction between
the block and the inclined plane is close to $${I \over {1000}}$$. The nearest integer to I is____.
4
When an object is kept at a distance of 30 cm from a concave mirror, the image is formed at a
distance of 10 cm from the mirror. If the object is moved with a speed of 9 cms–1, the speed
(in cms–1) with which image moves at that instant is ____.
5
A calorimeter of water equivalent 20 g contains 180 g of water at 25oC. ‘m’ grams of steam at
100oC is mixed in it till the temperature of the mixure is 31oC. The value of ‘m’ is close to :
(Latent heat of water = 540 cal g–1, specific heat of water = 1 cal g–1 oC–1)
(Latent heat of water = 540 cal g–1, specific heat of water = 1 cal g–1 oC–1)
6
Hydrogen ion and singly ionized helium atom are accelerated, from rest, through the same potential
difference. The ratio of final speeds of hydrogen and helium ions is close to :
7
Two light waves having the same wavelength $$\lambda $$ in vacuum are in phase initially. Then the first wave
travels a path L1
through a medium of refractive index n1
while the second wave travels a path of length
L2
through a medium of refractive index n2
. After this the phase difference between the two waves is :
8
The electric field of a plane electromagnetic wave propagating along the x direction in vacuum is
$$\overrightarrow E = {E_0}\widehat j\cos \left( {\omega t - kx} \right)$$.
The magnetic field $$\overrightarrow B $$ , at the moment t = 0 is :
$$\overrightarrow E = {E_0}\widehat j\cos \left( {\omega t - kx} \right)$$.
The magnetic field $$\overrightarrow B $$ , at the moment t = 0 is :
9
A perfectly diamagnetic sphere has a small spherical cavity at its centre, which is filled with a
paramagnetic substance. The whole system is placed in a uniform magnetic field $$\overrightarrow B $$
. Then the field
inside the paramagnetic substance is :


10
Concentric metallic hollow spheres of radii R and 4R hold charges Q1
and Q2
respectively. Given that
surface charge densities of the concentric spheres are equal, the potential difference V(R) – V(4R) is :
11
To raise the temperature of a certain mass of gas by 50oC at a constant pressure, 160 calories of
heat is required. When the same mass of gas is cooled by 100oC at constant volume, 240 calories
of heat is released. How many degrees of freedom does each molecule of this gas have (assume
gas to be ideal)?
12
A uniform magnetic field B exists in a direction perpendicular to the plane of a square loop made of
a metal wire. The wire has a diameter of 4 mm and a total length of 30 cm. The magnetic field
changes with time at a steady rate $${{dB} \over {dt}}$$ = 0.032 Ts–1. The induced current in the loop is close to
(Resistivity of the metal wire is 1.23 $$ \times $$ 10–8 $$\Omega $$m)
13
Amount of solar energy received on the earth’s surface per unit area per unit time is defined a solar
constant. Dimension of solar constant is :
14
The radius R of a nucleus of mass number A can be estimated by the formula
R = (1.3 $$ \times $$ 10–15)A1/3 m.
It follows that the mass density of a nucleus is of the order of :
(Mprot. $$ \cong $$ Mneut $$ \simeq $$ 1.67 $$ \times $$ 10–27 kg)
R = (1.3 $$ \times $$ 10–15)A1/3 m.
It follows that the mass density of a nucleus is of the order of :
(Mprot. $$ \cong $$ Mneut $$ \simeq $$ 1.67 $$ \times $$ 10–27 kg)
15
A block of mass 1.9 kg is at rest at the edge of a table, of height 1 m. A bullet of mass 0.1 kg
collides with the block and sticks to it. If the velocity of the bullet is 20 m/s in the horizontal
direction just before the collision then the kinetic energy just before the combined system strikes
the floor, is [Take g = 10 m/s2
. Assume there is no rotational motion and loss of energy after the
collision is negligable.]
16
Two resistors 400$$\Omega $$ and 800$$\Omega $$ are connected in series across a 6 V battery. The potential difference measured by a voltmeter of 10 k$$\Omega $$ across 400 $$\Omega $$ resistor is close to :
17
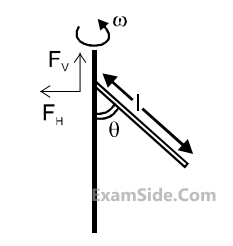
A uniform rod of length ‘$$l$$’ is pivoted at one of its ends on a vertical shaft of negligible radius.
When the shaft rotates at angular speed $$\omega $$ the rod makes an angle $$\theta $$ with it (see figure). To find $$\theta $$
equate the rate of change of angular momentum (direction going into the paper) $${{m{l^2}} \over {12}}{\omega ^2}\sin \theta \cos \theta $$
about the centre of mass (CM) to the torque provided by the horizontal and vertical forces FH
and
FV
about the CM. The value of $$\theta $$ is then such that :

18
Which of the following will NOT be observed when a multimeter (operating in resistance measuring
mode) probes connected across a component, are just reversed?
19
If a semiconductor photodiode can detect a photon with a maximum wavelength of 400 nm, then
its band gap energy is :
Planck’s constant h = 6.63 $$ \times $$ 10–34 J.s. Speed of light c = 3 $$ \times $$ 108 m/s
Planck’s constant h = 6.63 $$ \times $$ 10–34 J.s. Speed of light c = 3 $$ \times $$ 108 m/s
20
A particle is moving unidirectionally on a horizontal plane under the action of a constant power
supplying energy source. The displacement (s) - time (t) graph that describes the motion of the
particle is (graphs are drawn schematically and are not to scale) :
21
Two sources of light emit X-rays of wavelength 1 nm and visible light of wavelength 500 nm, respectively. Both the sources emit light of the same power 200 W. The ratio of the number density of
photons of X-rays to the number density of photons of the visible light of the given wavelengths is :
22
The mass density of a planet of radius R varies with the distance r from its centre as
$$\rho $$(r) = $${\rho _0}\left( {1 - {{{r^2}} \over {{R^2}}}} \right)$$.
Then the gravitational field is maximum at :
$$\rho $$(r) = $${\rho _0}\left( {1 - {{{r^2}} \over {{R^2}}}} \right)$$.
Then the gravitational field is maximum at :
23
A block of mass m attached to a massless spring is performing oscillatory motion of amplitude ‘A’ on
a frictionless horizontal plane. If half of the mass of the block breaks off when it is passing through
its equilibrium point, the amplitude of oscillation for the remaining system become fA. The value of
f is :