1
AIEEE 2012
MCQ (Single Correct Answer)
+4
-1
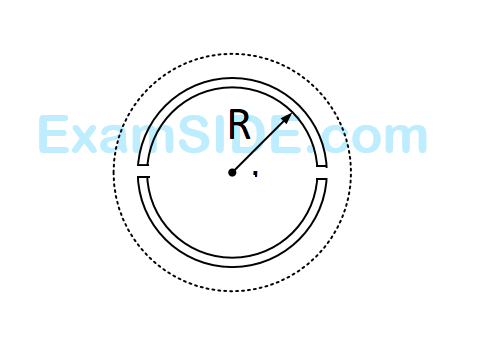
A wooden wheel of radius $$R$$ is made of two semicircular part (see figure). The two parts are held together by a ring made of a metal strip of cross sectional area $$S$$ and length $$L.$$ $$L$$ is slightly less than $$2\pi R.$$ To fit the ring on the wheel, it is heated so that its temperature rises by $$\Delta T$$ and it just steps over the wheel. As it cools down to surrounding temperature, it process the semicircular parts together. If the coefficient of linear expansion of the metal is $$\alpha $$, and its Young's modulus is $$Y,$$ the force that one part of the wheel applies on the other part is :

2
AIEEE 2012
MCQ (Single Correct Answer)
+4
-1
Out of Syllabus
A Carnot engine, whose efficiency is $$40\% $$, takes in heat from a source maintained at a temperature of $$500$$ $$K.$$ It is desired to have an engine of efficiency $$60\% .$$ Then, the intake temperature for the same exhaust (sink) temperature must be :
3
AIEEE 2011
MCQ (Single Correct Answer)
+4
-1
$$100g$$ of water is heated from $${30^ \circ }C$$ to $${50^ \circ }C$$. Ignoring the slight expansion of the water, the change in its internal energy is (specific heat of water is $$4184$$ $$J/kg/K$$):
4
AIEEE 2011
MCQ (Single Correct Answer)
+4
-1
Three perfect gases at absolute temperatures $${T_1},\,{T_2}$$ and $${T_3}$$ are mixed. The masses of molecules are $${m_1},{m_2}$$ and $${m_3}$$ and the number of molecules are $${n_1},$$ $${n_2}$$ and $${n_3}$$ respectively. Assuming no loss of energy, the final temperature of the mixture is:
Questions Asked from Heat and Thermodynamics (MCQ (Single Correct Answer))
Number in Brackets after Paper Indicates No. of Questions
JEE Main 2025 (Online) 8th April Evening Shift (2)
JEE Main 2025 (Online) 7th April Evening Shift (2)
JEE Main 2025 (Online) 7th April Morning Shift (1)
JEE Main 2025 (Online) 4th April Evening Shift (3)
JEE Main 2025 (Online) 4th April Morning Shift (2)
JEE Main 2025 (Online) 3rd April Evening Shift (2)
JEE Main 2025 (Online) 3rd April Morning Shift (3)
JEE Main 2025 (Online) 2nd April Evening Shift (2)
JEE Main 2025 (Online) 2nd April Morning Shift (2)
JEE Main 2025 (Online) 29th January Evening Shift (4)
JEE Main 2025 (Online) 29th January Morning Shift (1)
JEE Main 2025 (Online) 28th January Evening Shift (2)
JEE Main 2025 (Online) 28th January Morning Shift (2)
JEE Main 2025 (Online) 24th January Evening Shift (4)
JEE Main 2025 (Online) 24th January Morning Shift (1)
JEE Main 2025 (Online) 23rd January Evening Shift (2)
JEE Main 2025 (Online) 23rd January Morning Shift (2)
JEE Main 2025 (Online) 22nd January Evening Shift (2)
JEE Main 2025 (Online) 22nd January Morning Shift (2)
JEE Main 2024 (Online) 9th April Evening Shift (2)
JEE Main 2024 (Online) 9th April Morning Shift (2)
JEE Main 2024 (Online) 8th April Evening Shift (2)
JEE Main 2024 (Online) 8th April Morning Shift (2)
JEE Main 2024 (Online) 6th April Evening Shift (3)
JEE Main 2024 (Online) 6th April Morning Shift (2)
JEE Main 2024 (Online) 5th April Evening Shift (2)
JEE Main 2024 (Online) 5th April Morning Shift (2)
JEE Main 2024 (Online) 4th April Evening Shift (2)
JEE Main 2024 (Online) 4th April Morning Shift (3)
JEE Main 2024 (Online) 1st February Evening Shift (2)
JEE Main 2024 (Online) 1st February Morning Shift (2)
JEE Main 2024 (Online) 31st January Evening Shift (2)
JEE Main 2024 (Online) 31st January Morning Shift (2)
JEE Main 2024 (Online) 30th January Evening Shift (2)
JEE Main 2024 (Online) 30th January Morning Shift (2)
JEE Main 2024 (Online) 29th January Evening Shift (2)
JEE Main 2024 (Online) 29th January Morning Shift (2)
JEE Main 2024 (Online) 27th January Evening Shift (3)
JEE Main 2024 (Online) 27th January Morning Shift (2)
JEE Main 2023 (Online) 15th April Morning Shift (2)
JEE Main 2023 (Online) 13th April Evening Shift (2)
JEE Main 2023 (Online) 13th April Morning Shift (1)
JEE Main 2023 (Online) 12th April Morning Shift (2)
JEE Main 2023 (Online) 11th April Evening Shift (2)
JEE Main 2023 (Online) 11th April Morning Shift (3)
JEE Main 2023 (Online) 10th April Evening Shift (2)
JEE Main 2023 (Online) 10th April Morning Shift (2)
JEE Main 2023 (Online) 8th April Evening Shift (2)
JEE Main 2023 (Online) 8th April Morning Shift (1)
JEE Main 2023 (Online) 6th April Evening Shift (3)
JEE Main 2023 (Online) 6th April Morning Shift (2)
JEE Main 2023 (Online) 1st February Evening Shift (2)
JEE Main 2023 (Online) 1st February Morning Shift (3)
JEE Main 2023 (Online) 31st January Evening Shift (2)
JEE Main 2023 (Online) 31st January Morning Shift (2)
JEE Main 2023 (Online) 30th January Evening Shift (2)
JEE Main 2023 (Online) 30th January Morning Shift (2)
JEE Main 2023 (Online) 29th January Evening Shift (2)
JEE Main 2023 (Online) 29th January Morning Shift (1)
JEE Main 2023 (Online) 25th January Evening Shift (3)
JEE Main 2023 (Online) 25th January Morning Shift (2)
JEE Main 2023 (Online) 24th January Evening Shift (2)
JEE Main 2023 (Online) 24th January Morning Shift (2)
JEE Main 2022 (Online) 29th July Evening Shift (2)
JEE Main 2022 (Online) 28th July Evening Shift (1)
JEE Main 2022 (Online) 28th July Morning Shift (2)
JEE Main 2022 (Online) 27th July Evening Shift (2)
JEE Main 2022 (Online) 27th July Morning Shift (3)
JEE Main 2022 (Online) 26th July Evening Shift (2)
JEE Main 2022 (Online) 26th July Morning Shift (2)
JEE Main 2022 (Online) 25th July Evening Shift (2)
JEE Main 2022 (Online) 25th July Morning Shift (2)
JEE Main 2022 (Online) 30th June Morning Shift (2)
JEE Main 2022 (Online) 29th June Evening Shift (3)
JEE Main 2022 (Online) 29th June Morning Shift (2)
JEE Main 2022 (Online) 28th June Evening Shift (2)
JEE Main 2022 (Online) 28th June Morning Shift (1)
JEE Main 2022 (Online) 27th June Evening Shift (3)
JEE Main 2022 (Online) 27th June Morning Shift (1)
JEE Main 2022 (Online) 26th June Evening Shift (1)
JEE Main 2022 (Online) 26th June Morning Shift (2)
JEE Main 2022 (Online) 25th June Evening Shift (3)
JEE Main 2022 (Online) 25th June Morning Shift (1)
JEE Main 2022 (Online) 24th June Evening Shift (2)
JEE Main 2022 (Online) 24th June Morning Shift (2)
JEE Main 2021 (Online) 31st August Evening Shift (2)
JEE Main 2021 (Online) 31st August Morning Shift (2)
JEE Main 2021 (Online) 27th August Evening Shift (2)
JEE Main 2021 (Online) 27th August Morning Shift (2)
JEE Main 2021 (Online) 26th August Evening Shift (3)
JEE Main 2021 (Online) 26th August Morning Shift (2)
JEE Main 2021 (Online) 27th July Evening Shift (2)
JEE Main 2021 (Online) 27th July Morning Shift (2)
JEE Main 2021 (Online) 25th July Evening Shift (1)
JEE Main 2021 (Online) 25th July Morning Shift (3)
JEE Main 2021 (Online) 22th July Evening Shift (1)
JEE Main 2021 (Online) 20th July Evening Shift (2)
JEE Main 2021 (Online) 20th July Morning Shift (3)
JEE Main 2021 (Online) 18th March Evening Shift (3)
JEE Main 2021 (Online) 18th March Morning Shift (2)
JEE Main 2021 (Online) 17th March Evening Shift (2)
JEE Main 2021 (Online) 17th March Morning Shift (4)
JEE Main 2021 (Online) 16th March Evening Shift (2)
JEE Main 2021 (Online) 16th March Morning Shift (2)
JEE Main 2021 (Online) 26th February Evening Shift (1)
JEE Main 2021 (Online) 26th February Morning Shift (1)
JEE Main 2021 (Online) 25th February Evening Shift (2)
JEE Main 2021 (Online) 25th February Morning Shift (2)
JEE Main 2021 (Online) 24th February Evening Shift (2)
JEE Main 2021 (Online) 24th February Morning Shift (3)
JEE Main 2020 (Online) 6th September Evening Slot (2)
JEE Main 2020 (Online) 6th September Morning Slot (1)
JEE Main 2020 (Online) 5th September Evening Slot (2)
JEE Main 2020 (Online) 5th September Morning Slot (3)
JEE Main 2020 (Online) 4th September Evening Slot (1)
JEE Main 2020 (Online) 4th September Morning Slot (2)
JEE Main 2020 (Online) 3rd September Evening Slot (2)
JEE Main 2020 (Online) 3rd September Morning Slot (2)
JEE Main 2020 (Online) 2nd September Evening Slot (3)
JEE Main 2020 (Online) 2nd September Morning Slot (1)
JEE Main 2020 (Online) 9th January Evening Slot (1)
JEE Main 2020 (Online) 9th January Morning Slot (2)
JEE Main 2020 (Online) 8th January Evening Slot (2)
JEE Main 2020 (Online) 8th January Morning Slot (2)
JEE Main 2020 (Online) 7th January Evening Slot (2)
JEE Main 2020 (Online) 7th January Morning Slot (2)
JEE Main 2019 (Online) 12th April Evening Slot (3)
JEE Main 2019 (Online) 12th April Morning Slot (4)
JEE Main 2019 (Online) 10th April Evening Slot (2)
JEE Main 2019 (Online) 10th April Morning Slot (3)
JEE Main 2019 (Online) 9th April Evening Slot (3)
JEE Main 2019 (Online) 9th April Morning Slot (3)
JEE Main 2019 (Online) 8th April Evening Slot (2)
JEE Main 2019 (Online) 8th April Morning Slot (2)
JEE Main 2019 (Online) 12th January Evening Slot (2)
JEE Main 2019 (Online) 12th January Morning Slot (3)
JEE Main 2019 (Online) 11th January Evening Slot (5)
JEE Main 2019 (Online) 11th January Morning Slot (3)
JEE Main 2019 (Online) 10th January Evening Slot (3)
JEE Main 2019 (Online) 10th January Morning Slot (2)
JEE Main 2019 (Online) 9th January Evening Slot (2)
JEE Main 2019 (Online) 9th January Morning Slot (4)
JEE Main 2018 (Online) 16th April Morning Slot (2)
JEE Main 2018 (Offline) (1)
JEE Main 2018 (Online) 15th April Evening Slot (2)
JEE Main 2018 (Online) 15th April Morning Slot (2)
JEE Main 2017 (Online) 9th April Morning Slot (3)
JEE Main 2017 (Online) 8th April Morning Slot (4)
JEE Main 2017 (Offline) (4)
JEE Main 2016 (Online) 10th April Morning Slot (2)
JEE Main 2016 (Online) 9th April Morning Slot (3)
JEE Main 2016 (Offline) (3)
JEE Main 2015 (Offline) (3)
JEE Main 2014 (Offline) (2)
JEE Main 2013 (Offline) (2)
AIEEE 2012 (3)
AIEEE 2011 (4)
AIEEE 2010 (1)
AIEEE 2009 (6)
AIEEE 2008 (2)
AIEEE 2007 (4)
AIEEE 2006 (3)
AIEEE 2005 (4)
AIEEE 2004 (6)
AIEEE 2003 (5)
AIEEE 2002 (10)
JEE Main Subjects
Physics
Mechanics
Units & Measurements Vector Algebra Motion in a Straight Line Motion in a Plane Circular Motion Laws of Motion Work Power & Energy Center of Mass and Collision Rotational Motion Properties of Matter Heat and Thermodynamics Simple Harmonic Motion Waves Gravitation
Electricity
Electrostatics Current Electricity Capacitor Magnetic Effect of Current Magnetic Properties of Matter Electromagnetic Induction Alternating Current Electromagnetic Waves
Optics
Modern Physics
Chemistry
Physical Chemistry
Some Basic Concepts of Chemistry Structure of Atom Redox Reactions Gaseous State Chemical Equilibrium Ionic Equilibrium Solutions Thermodynamics Electrochemistry Chemical Kinetics and Nuclear Chemistry Solid State Surface Chemistry
Inorganic Chemistry
Periodic Table & Periodicity Chemical Bonding & Molecular Structure Isolation of Elements Hydrogen s-Block Elements p-Block Elements d and f Block Elements Coordination Compounds Salt Analysis Environmental Chemistry
Organic Chemistry
Mathematics
Algebra
Sets and Relations Logarithm Quadratic Equation and Inequalities Sequences and Series Mathematical Induction Binomial Theorem Matrices and Determinants Permutations and Combinations Probability Vector Algebra 3D Geometry Complex Numbers Statistics Mathematical Reasoning
Trigonometry
Trigonometric Ratio and Identites Trigonometric Equations Inverse Trigonometric Functions Properties of Triangle Height and Distance
Coordinate Geometry
Calculus