
In a reaction $A+B \rightarrow C$, initial concentrations of $A$ and $B$ are related as $[A]_0=8[B]_0$. The half lives of $A$ and $B$ are 10 min and 40 min , respectively. If they start to disappear at the same time, both following first order kinetics, after how much time will the concentration of both the reactants be same?

Number of molecules from below which cannot give iodoform reaction is :
Ethanol, Isopropyl alcohol, Bromoacetone, 2-Butanol, 2-Butanone, Butanal, 2-Pentanone, 3-Pentanone, Pentanal and 3-Pentanol.

$$ \text {During estimation of nitrogen by Dumas' method of compound } \mathrm{X}(0.42 \mathrm{~g}) $$
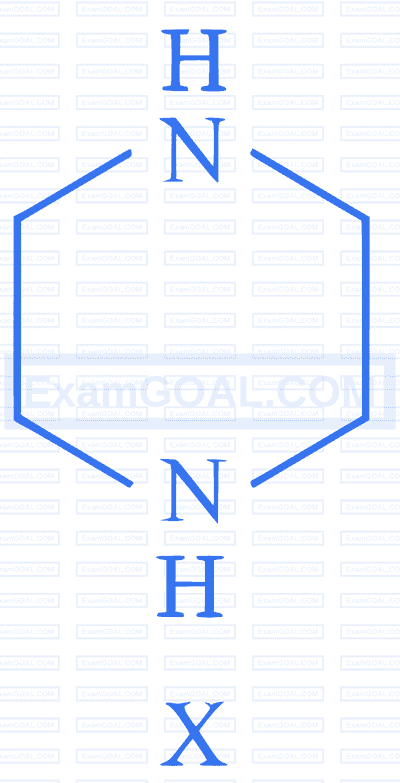
_________mL of $\mathrm{N}_2$ gas will be liberated at STP. (nearest integer)
(Given molar mass in $\mathrm{g}~ \mathrm{mol}^{-1}: \mathrm{C}: 12, \mathrm{H}: 1, \mathrm{~N}: 14$ )
Consider the following reactions
$$ \begin{aligned} & \mathrm{A}+\underset{\substack{ \text { Little } \\ \text { amount }}}{\mathrm{NaCl}}+\mathrm{H}_2 \mathrm{SO}_4 \rightarrow \mathrm{CrO}_2 \mathrm{Cl}_2+\text { Side Products } \\ & \mathrm{CrO}_2 \mathrm{Cl}_{2 \text { (Vapour) }}+\mathrm{NaOH} \rightarrow \mathrm{~B}+\mathrm{NaCl}+\mathrm{H}_2 \mathrm{O} \\ & \mathrm{~B}+\mathrm{H}^{+} \rightarrow \mathrm{C}+\mathrm{H}_2 \mathrm{O} \end{aligned} $$
The number of terminal ' $O$ ' present in the compound ' C ' is__________