
X g of nitrobenzene on nitration gave 4.2 g of m -dinitrobenzene. X = __________g. (nearest integer)
[Given : molar mass (in $\left.\left.\mathrm{g} \mathrm{mol}^{-1}\right) \mathrm{C}: 12, \mathrm{H}: 1, \mathrm{O}: 16, \mathrm{~N}: 14\right]$

$$ \text {During estimation of nitrogen by Dumas' method of compound } \mathrm{X}(0.42 \mathrm{~g}) $$
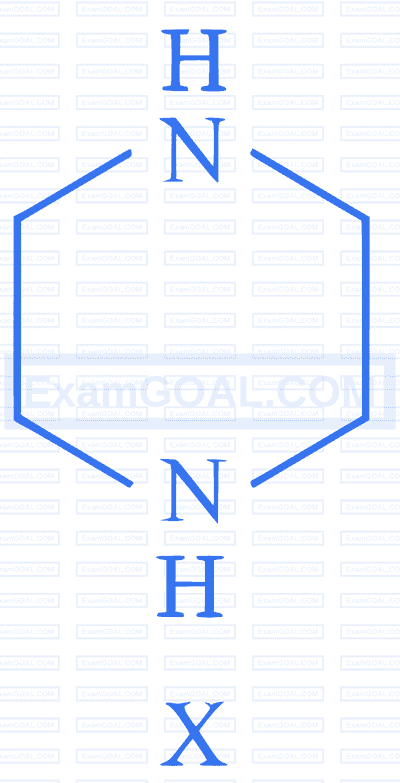
_________mL of $\mathrm{N}_2$ gas will be liberated at STP. (nearest integer)
(Given molar mass in $\mathrm{g}~ \mathrm{mol}^{-1}: \mathrm{C}: 12, \mathrm{H}: 1, \mathrm{~N}: 14$ )
0.5 g of an organic compound on combustion gave 1.46 g of $\mathrm{CO}_2$ and 0.9 g of $\mathrm{H}_2 \mathrm{O}$. The percentage of carbon in the compound is _______________. (Nearest integer)
[Given : Molar mass (in $\left.\mathrm{g} \mathrm{mol}^{-1}\right) \mathrm{C}: 12, \mathrm{H}: 1, \mathrm{O}: 16$ ]

The molarity of a $70 \%$ (mass/mass) aqueous solution of a monobasic acid (X) is _________ $\times 10^{-1}$ M (Nearest integer)
[Given: Density of aqueous solution of (X) is $1.25 \mathrm{~g} \mathrm{~mL}^{-1}$
Molar mass of the acid is $70 \mathrm{~g} \mathrm{~mol}^{-1}$ ]