
The amount of calcium oxide produced on heating 150 kg limestone ( $75 \%$ pure) is _________ kg. (Nearest integer)
Given: Molar mass (in $\mathrm{g} \mathrm{mol}^{-1}$ ) of Ca-40, O-16, C-12

Fortification of food with iron is done using $\mathrm{FeSO}_4 \cdot 7 \mathrm{H}_2 \mathrm{O}$. The mass in grams of the $\mathrm{FeSO}_4$. $7 \mathrm{H}_2 \mathrm{O}$ required to achieve 12 ppm of iron in 150 kg of wheat is ______ (Nearest integer)
[Given: Molar mass of $\mathrm{Fe}, \mathrm{S}$ and and O respectively are 56, 32 and $16 \mathrm{~g} \mathrm{~mol}^{-1}$ ]

X g of nitrobenzene on nitration gave 4.2 g of m -dinitrobenzene. X = __________g. (nearest integer)
[Given : molar mass (in $\left.\left.\mathrm{g} \mathrm{mol}^{-1}\right) \mathrm{C}: 12, \mathrm{H}: 1, \mathrm{O}: 16, \mathrm{~N}: 14\right]$

$$ \text {During estimation of nitrogen by Dumas' method of compound } \mathrm{X}(0.42 \mathrm{~g}) $$
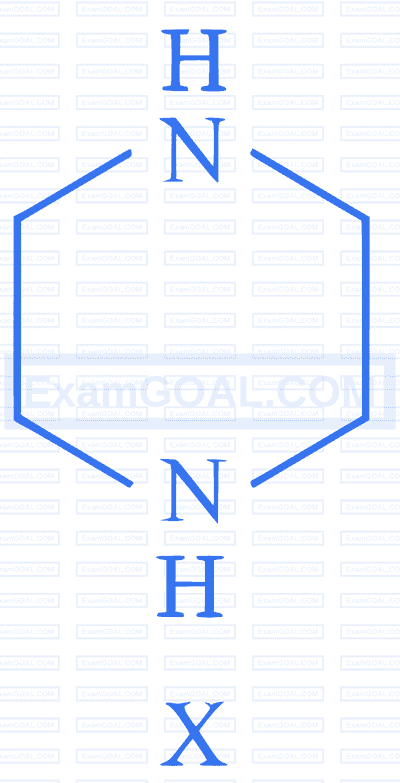
_________mL of $\mathrm{N}_2$ gas will be liberated at STP. (nearest integer)
(Given molar mass in $\mathrm{g}~ \mathrm{mol}^{-1}: \mathrm{C}: 12, \mathrm{H}: 1, \mathrm{~N}: 14$ )