
The extra stability of half-filled subshell is due to :
(A) Symmetrical distribution of electrons
(B) Smaller coulombic repulsion energy
(C) The presence of electrons with the same spin in non-degenerate orbitals
(D) Larger exchange energy
(E) Relatively smaller shielding of electrons by one another
Indentify the correct statements :

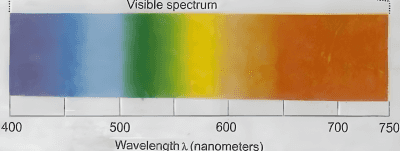
Which of the following statements are correct, if the threshold frequency of caesium is $5.16 \times$ $10^{14} \mathrm{~Hz}$ ?
A. When Cs is placed inside a vacuum chamber with an ammeter connected to it and yellow light is focused on Cs , the ammeter shows the presence of current.
B. When the brightness of the yellow light is dimmed, the value of the current in the ammeter is reduced.
C. When a red light is used instead of the yellow light, the current produced is higher with respect to the yellow light.
D. When a blue light is used, the ammeter shows the formation of current.
E. When a white light is used. the ammeter shows formation of current.
Choose the correct answer from the options given below:

Consider the ground state of chromium atom $(Z=24)$. How many electrons are with Azimuthal quantum number $l=1$ and $l=2$ respectively ?

Which one of the following about an electron occupying the 1 s orbital in a hydrogen atom is incorrect?
(Bohr's radius is represented by $\mathrm{a}_0$)