
Consider the following volume-temperature $(\mathrm{V}-\mathrm{T})$ diagram for the expansion of 5 moles of an ideal monoatomic gas.
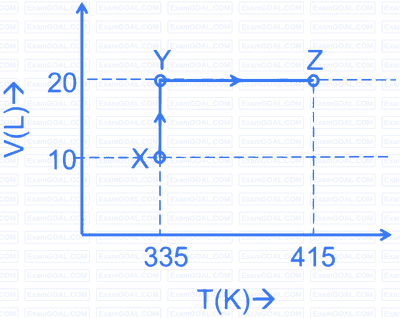
Considering only $\mathrm{P}-\mathrm{V}$ work is involved, the total change in enthalpy (in Joule) for the transformation of state in the sequence $\mathbf{X} \rightarrow \mathbf{Y} \rightarrow \mathbf{Z}$ is ____________.
[Use the given data: Molar heat capacity of the gas for the given temperature range, $\mathrm{C}_{\mathrm{V}, \mathrm{m}}=12 \mathrm{~J} \mathrm{~K}^{-1}$ $\mathrm{mol}^{-1}$ and gas constant, $\left.\mathrm{R}=8.3 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}\right]$

Consider the following reaction,
$$ 2 \mathrm{H}_2(\mathrm{~g})+2 \mathrm{NO}(\mathrm{g}) \rightarrow \mathrm{N}_2(\mathrm{~g})+2 \mathrm{H}_2 \mathrm{O}(\mathrm{g}) $$
which follows the mechanism given below :
$$ \begin{array}{ll} 2 \mathrm{NO}(\mathrm{g}) \stackrel{k_1}{\underset{k_{-1}}{\rightleftharpoons}} \mathrm{N}_2 \mathrm{O}_2(\mathrm{~g}) & \text { (fast equlibrium) } \\\\ \mathrm{N}_2 \mathrm{O}_2(\mathrm{~g})+\mathrm{H}_2(\mathrm{~g}) \xrightarrow{k_2} \mathrm{~N}_2 \mathrm{O}(\mathrm{g})+\mathrm{H}_2 \mathrm{O}(\mathrm{g}) & \text { (slow reaction) } \\\\ \mathrm{N}_2 \mathrm{O}(\mathrm{g})+\mathrm{H}_2(\mathrm{~g}) \xrightarrow{k_3} \mathrm{~N}_2(\mathrm{~g})+\mathrm{H}_2 \mathrm{O}(\mathrm{g}) & \text { (fast reaction) } \end{array} $$
The order of the reaction is __________.

Complete reaction of acetaldehyde with excess formaldehyde, upon heating with conc. $\mathrm{NaOH}$ solution, gives $\mathbf{P}$ and $\mathbf{Q}$. Compound $\mathbf{P}$ does not give Tollens' test, whereas $\mathbf{Q}$ on acidification gives positive Tollens' test. Treatment of $\mathbf{P}$ with excess cyclohexanone in the presence of catalytic amount of $p$-toluenesulfonic acid (PTSA) gives product $\mathbf{R}$.
Sum of the number of methylene groups $\left(-\mathrm{CH}_2-\right)$ and oxygen atoms in $\mathbf{R}$ is __________.

Among $\mathrm{V}(\mathrm{CO})_6, \mathrm{Cr}(\mathrm{CO})_5, \mathrm{Cu}(\mathrm{CO})_3, \mathrm{Mn}(\mathrm{CO})_5, \mathrm{Fe}(\mathrm{CO})_5,\left[\mathrm{Co}(\mathrm{CO})_3\right]^{3-},\left[\mathrm{Cr}(\mathrm{CO})_4\right]^{4-}$, and $\operatorname{Ir}(\mathrm{CO})_3$, the total number of species isoelectronic with $\mathrm{Ni}(\mathrm{CO})_4$ is _________.
[Given, atomic number: $\mathrm{V}=23, \mathrm{Cr}=24, \mathrm{Mn}=25, \mathrm{Fe}=26, \mathrm{Co}=27, \mathrm{Ni}=28, \mathrm{Cu}=29, \mathrm{Ir}=77$ ]