Chemistry
9Be4 + X $$\to$$ 8Be4 + Y
(X, Y) is (are) :
List - I
P. $$\mathop {(C{}_2{H_5}){}_3N}\limits_X $$ + $$\mathop {C{H_3}COOH}\limits_Y $$
Q. $$\mathop {KI(0.1M)}\limits_X $$ + $$\mathop {AgN{O_3}(0.01M)}\limits_Y $$
R. $$\mathop {C{H_3}COOH}\limits_X $$ + $$\mathop {KOH}\limits_Y $$
S. $$\mathop {NaOH}\limits_X $$ + $$\mathop {HI}\limits_Y $$
List - II
1. Conductivity decreases then increases
2. Conductivity decreases then does not change much
3. Conductivity increases then does not change much
4. Conductivity does not change much then increases
Eo (Fe3+ , Fe2+) = +0.77V;
Eo (Fe2+ , Fe) = -0.44V;
Eo (Cu2+ , Cu) = +0.34V;
Eo (Cu+ , Cu) = +0.52V;
Eo [O2(g) + 4H+ + 4e- $$\to$$ 2H2O] = +1.23V;
Eo [O2(g) + 2H2O + 4e- $$\to$$ 4OH-] = +0.40 V
Eo (Cr3+ , Cr) = -0.74V;
Eo (Cr2+ , Cr) = -0.91V;
Match Eo of the redox pair in List – I with the values given in List – II and select the correct answer using the code given below the lists:
List - I
P. Eo (Fe3+ , Fe)
Q. Eo (4H2O $$\leftrightharpoons$$ 4H+ + 4OH-)
R. Eo (Cu2+ + Cu $$\to$$ 2Cu+)
S. Eo (Cr3+, Cr2+)
List - II
1. -0.18 V
2. -0.4 V
3. -0.04 V
4. -0.83 V
CaCO3(s) $$\leftrightharpoons$$ CaO(s) + CO2(g).
For this equilibrium, the correct statement(s) is (are)
In the following reaction, the product/products formed is/are

The major product(s) of the following reaction is(are)

After completion of the reactions (I and II), the organic compound(s) in the reaction mixtures is(are)

The correct statement(s) about O3 is(are)
The precipitate P contains
The coloured solution S contains
Compounds formed from P and Q are, respectively,
In the following reaction sequences V and W are, respectively,

The succeeding operations that enable this transformation of states are
The pair of isochoric processes among the transformation of states is
R, S and T respectively, are
The unbalanced chemical reactions given in List I show missing reagent or condition (?) which are provided in List II. Match List I with List II and select the correct answer using the code given below the lists :
| List I | List II | ||
|---|---|---|---|
| P. | $$Pb{O_2} + {H_2}S{O_4}\buildrel ? \over \longrightarrow PbS{O_4} + {O_2} + Other\,products$$ |
1. | NO |
| Q. | $$N{a_2}{S_2}{O_3} + {H_2}O\buildrel ? \over \longrightarrow NaHS{O_4} + Other\,products$$ |
2. | $${I_2}$$ |
| R. | $${N_2}{H_4}\buildrel ? \over \longrightarrow {N_2} + Other\,products$$ |
3. | Warm |
| S. | $$Xe{F_2}\buildrel ? \over \longrightarrow Xe + Other\,products$$ |
4. | $$C{l_2}$$ |
Match the chemical conversions in List I with the appropriate reagents in List II and select the correct answer using the code given below the lists :

Mathematics
$$\,\,\,\,$$ $$\,\,\,\,$$ $$\,\,\,\,$$ List $$I$$
(P.)$$\,\,\,\,$$ Volume of parallelopiped determined by vectors $$\overrightarrow a ,\overrightarrow b $$ and $$\overrightarrow c $$ is $$2.$$ Then the volume of the parallelepiped determined by vectors $$2\left( {\overrightarrow a \times \overrightarrow b } \right),3\left( {\overrightarrow b \times \overrightarrow c } \right)$$ and $$\left( {\overrightarrow c \times \overrightarrow a } \right)$$ is
(Q.)$$\,\,\,\,$$ Volume of parallelopiped determined by vectors $$\overrightarrow a ,\overrightarrow b $$ and $$\overrightarrow c $$ is $$5.$$ Then the volume of the parallelepiped determined by vectors $$3\left( {\overrightarrow a + \overrightarrow b } \right),\left( {\overrightarrow b + \overrightarrow c } \right)$$ and $$2\left( {\overrightarrow c + \overrightarrow a } \right)$$ is
(R.)$$\,\,\,\,$$ Area of a triangle with adjacent sides determined by vectors $${\overrightarrow a }$$ and $${\overrightarrow b }$$ is $$20.$$ Then the area of the triangle with adjacent sides determined by vectors $$\left( {2\overrightarrow a + 3\overrightarrow b } \right)$$ and $$\left( {\overrightarrow a - \overrightarrow b } \right)$$ is
(S.)$$\,\,\,\,$$ Area of a parallelogram with adjacent sides determined by vectors $${\overrightarrow a }$$ and $${\overrightarrow b }$$ is $$30.$$ Then the area of the parallelogram with adjacent sides determined by vectors $$\left( {\overrightarrow a + \overrightarrow b } \right)$$ and $${\overrightarrow a }$$ is
$$\,\,\,\,$$ $$\,\,\,\,$$ $$\,\,\,\,$$ List $$II$$
(1.)$$\,\,\,\,$$ $$100$$
(2.)$$\,\,\,\,$$ $$30$$
(3.)$$\,\,\,\,$$ $$24$$
(4.)$$\,\,\,\,$$ $$60$$
Let $\omega=\frac{\sqrt{3}+i}{2}$ and $P=\left\{\omega^n: n=1,2,3, \ldots\right\}$. Further
$\mathrm{H}_1=\left\{z \in \mathrm{C}: \operatorname{Re} z<\frac{1}{2}\right\}$ and
$\mathrm{H}_2=\left\{z \in \mathrm{C}: \operatorname{Re} z<\frac{-1}{2}\right\}$, where C is the
set of all complex numbers. If $z_1 \in \mathrm{P} \cap \mathrm{H}_1, z_2 \in$ $\mathrm{P} \cap \mathrm{H}_2$ and O
represents the origin, then $\angle z_1 \mathrm{O} z_2=$
$${L_1}:{{x - 1} \over 2} = {y \over { - 1}} = {{z + 3} \over 1},{L_2} : {{x - 4} \over 1} = {{y + 3} \over 1} = {{z + 3} \over 2}$$
and the planes $${P_1}:7x + y + 2z = 3,{P_2} = 3x + 5y - 6z = 4.$$ Let $$ax+by+cz=d$$ be the equation of the plane passing through the point of intersection of lines $${L_1}$$ and $${L_2},$$ and perpendicular to planes $${P_1}$$ and $${P_2}.$$
Match List $$I$$ with List $$II$$ and select the correct answer using the code given below the lists:
List $$I$$
(P.) $$a=$$
(Q.) $$b=$$
(R.) $$c=$$
(S.) $$d=$$
List $$II$$
(1.) $$13$$
(2.) $$-3$$
(3.) $$1$$
(4.) $$-2$$
If $$1$$ ball is drawn from each of the boxex $${B_1},$$ $${B_2}$$ and $${B_3},$$ the probability that all $$3$$ drawn balls are of the same colour is
If $$2$$ balls are drawn (without replacement) from a randomly selected box and one of the balls is white and the other ball is red, the probability that these $$2$$ balls are drawn from box $${B_2}$$ is
$$f(0) = f(1)=0$$ and satisfies $$f''\left( x \right) - 2f'\left( x \right) + f\left( x \right) \ge .{e^x},x \in \left[ {0,1} \right]$$.
If the function $${e^{ - x}}f\left( x \right)$$ assumes its minimum in the interval $$\left[ {0,1} \right]$$ at $$x = {1 \over 4}$$, which of the following is true?
$$f(0) = f(1)=0$$ and satisfies $$f''\left( x \right) - 2f'\left( x \right) + f\left( x \right) \ge .{e^x},x \in \left[ {0,1} \right]$$.
Which of the following is true for $$0 < x < 1?$$
List $$I$$
$$P.$$$$\,\,\,\,\,$$ $${\left( {{1 \over {{y^2}}}{{\left( {{{\cos \left( {{{\tan }^{ - 1}}y} \right) + y\sin \left( {{{\tan }^{ - 1}}y} \right)} \over {\cot \left( {{{\sin }^{ - 1}}y} \right) + \tan \left( {{{\sin }^{ - 1}}y} \right)}}} \right)}^2} + {y^4}} \right)^{1/2}}$$ takes value
$$Q.$$ $$\,\,\,\,$$ If $$\cos x + \cos y + \cos z = 0 = \sin x + \sin y + \sin z$$ then
possible value of $$\cos {{x - y} \over 2}$$ is
$$R.$$ $$\,\,\,\,\,$$ If $$\cos \left( {{\pi \over 4} - x} \right)\cos 2x + \sin x\sin 2\sec x = \cos x\sin 2x\sec x + $$
$$\cos \left( {{\pi \over 4} + x} \right)\cos 2x$$ then possible value of $$\sec x$$ is
$$S.$$ $$\,\,\,\,\,$$ If $$\cot \left( {{{\sin }^{ - 1}}\sqrt {1 - {x^2}} } \right) = \sin \left( {{{\tan }^{ - 1}}\left( {x\sqrt 6 } \right)} \right),\,\,x \ne 0,$$
Then possible value of $$x$$ is
List $$II$$
$$1.$$ $$\,\,\,\,\,$$ $${1 \over 2}\sqrt {{5 \over 3}} $$
$$2.$$ $$\,\,\,\,\,$$ $$\sqrt 2 $$
$$3.$$ $$\,\,\,\,\,$$ $${1 \over 2}$$
$$1.$$ $$\,\,\,\,$$ $$1$$
If chord $$PQ$$ subtends an angle $$\theta $$ at the vertex of $${y^2} = 4ax$$, then tan $$\theta = $$
Length of chord $$PQ$$ is
Match List $$I$$ with List $$II$$ and select the correct answer using the code given below the lists:
List $$I$$
P.$$\,\,\,m = $$
Q.$$\,\,\,$$Maximum area of $$\Delta EFG$$ is
R.$$\,\,\,$$ $${y_0} = $$
S.$$\,\,\,$$ $${y_1} = $$
List $$II$$
1.$$\,\,\,$$ $${1 \over 2}$$
2.$$\,\,\,$$ $$4$$
3.$$\,\,\,$$ $$2$$
4.$$\,\,\,$$ $$1$$
$$\,\mathop {\min }\limits_{z \in S} \left| {1 - 3i - z} \right| = $$
Area of S =
$$a \in R$$ (the set of all real numbers), a $$\ne$$ $$-$$1,
$$\mathop {\lim }\limits_{n \to \infty } {{({1^a} + {2^a} + ... + {n^a})} \over {{{(n + 1)}^{a - 1}}[(na + 1) + (na + 2) + ... + (na + n)]}} = {1 \over {60}}$$, Then a = ?
Let $$\omega$$ be a complex cube root of unity with $$\omega$$ $$\ne$$ 1 and P = [pij] be a n $$\times$$ n matrix with pij = $$\omega$$i + j. Then P2 $$\ne$$ 0, when n = ?
The function $$f(x) = 2\left| x \right| + \left| {x + 2} \right| - \left| {\left| {x + 2} \right| - 2\left| x \right|} \right|$$ has a local minimum or a local maximum at x =
Physics
The magnitude of the normal reaction that acts on the block at the point Q is
The speed of the block when it reaches the point Q is
List I
P. Boltzmann ConstantQ. Coefficient of viscosity
R. Plank Constant
S. Thermal conductivity
List II
1. [ML2T-1]2. [ML-1T-1]
3. [MLT-3K-1]
4. [ML2T-2K-1]
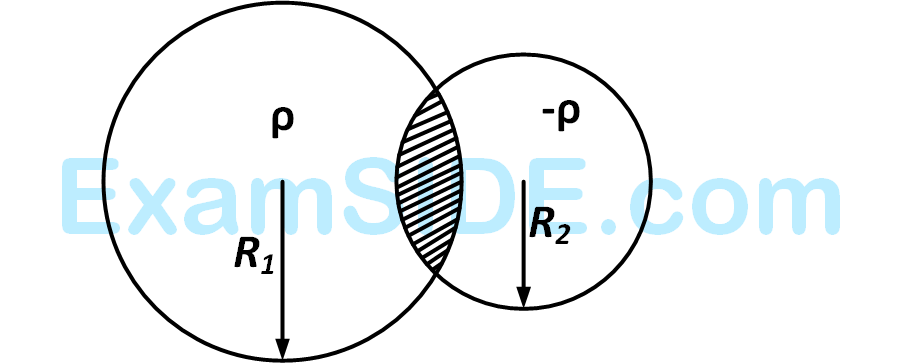
A steady current I flows along an infinitely long hollow cylindrical conductor of radius R. This cylinder is placed coaxially inside an infinite solenoid of radius 2R. The solenoid has n turns per unit length and carries a steady current I. Consider a point P at a distance r from the common axis. The correct statement(s) is(are)
Two vehicles, each moving with speed u on the same horizontal straight road, are approaching each other. Wind blows along the road with velocity w. One of these vehicles blows a whistle of frequency f1. An observer in the other vehicle hears the frequency of the whistle to be f2. The speed of sound in still air is V. The correct statement(s) is(are)
The figure below shows the variation of specific heat capacity (C) of a solid as a function of temperature (T). The temperature is increased continuously from 0 to 500 K at a constant rate. Ignoring any volume change, the following statement(s) is(are) correct to a reasonable approximation.

The radius of the orbit of an electron in a hydrogen-like atom is 4.5a0, where a0 is the Bohr radius. Its orbital angular momentum is $${{3h} \over {2\pi }}$$. It is given that h is Planck constant and R is Rydberg constant. The possible wavelength(s), when the atom de-excites, is(are)
If the direct transmission method with a cable of resistance 0.4 $$\Omega$$ km$$-$$1 is used, the power dissipation (in %) during transmission is
In the method using the transformers, assume that the ratio of the number of turns in the primary to that in the secondary in the step-up transformer is 1 : 10. If the power of the consumers has to be supplied at 200 V, the ratio of the number of turns in the primary to that in the secondary in the step-down transformer is
The change in the magnetic dipole moment associated with the orbit, at the end of the time interval of the magnetic field change, is
The correct statement is
The kinetic energy (in keV) of the alpha particle, when the nucleus $$_{84}^{210}Po$$ at rest undergoes alpha decay, is
A right-angled prism of refractive index $$\mu$$1 is placed in a rectangular block of refractive index $$\mu$$2, which is surrounded by a medium of refractive index $$\mu$$3, as shown in the figure. A ray of light e enters the rectangular block at normal incidence. Depending upon the relationships between $$\mu$$1, $$\mu$$2 and $$\mu$$3, it takes one of the four possible paths 'ef', 'eg', 'eh' or 'ei'.

Match the paths in List I with conditions of refractive indices in List II and select the correct answer using the codes given below the lists:
| List I | List II | ||
|---|---|---|---|
| P. | $$e \to f$$ |
1. | $${\mu _1} > \sqrt 2 {\mu _2}$$ |
| Q. | $$e \to g$$ |
2. | $${\mu _2} > {\mu _1}$$ and $${\mu _2} > {\mu _3}$$ |
| R. | $$e \to h$$ |
3. | $${\mu _1} = {\mu _2}$$ |
| S. | $$e \to i$$ |
4. | $${\mu _2} < {\mu _1} < \sqrt 2 {\mu _2}$$ and $${\mu _2} > {\mu _3}$$ |
One mole of a monatomic ideal gas is taken along two cyclic processes E $$\to$$ F $$\to$$ G $$\to$$ E and E $$\to$$ F $$\to$$ H $$\to$$ E as shown in the PV diagram. The processes involved are purely isochoric, isobaric, isothermal or adiabatic.

Match the paths in List I with the magnitudes of the work done in List II and select the correct answer using the codes given below the lists :
| List I | List II | ||
|---|---|---|---|
| P. | $$G \to E$$ |
1. | 160$${P_0}{V_0}$$ln2 |
| Q. | $$G \to H$$ |
2. | 36$${P_0}{V_0}$$ |
| R. | $$F \to H$$ |
3. | 24$${P_0}{V_0}$$ |
| S. | $$F \to G$$ |
4. | 31$${P_0}{V_0}$$ |
Match List I of the nuclear processes with List II containing parent nucleus and one of the end products of each process and then select the correct answer using the codes given below the lists :
| List I | List II | ||
|---|---|---|---|
| P. | Alpha decay | 1. | $$_8^{15}O \to _7^{15}N + ...$$ |
| Q. | $${\beta ^ + }$$ decay | 2. | $$_{91}^{238}U \to _{90}^{234}Th + ...$$ |
| R. | Fission | 3. | $$_{83}^{185}Bi \to _{82}^{184}Pb + ...$$ |
| S. | Proton emission | 4. | $$_{94}^{239}Pu \to _{57}^{140}La + ...$$ |