
Among the following, the correct statement(s) for electrons in an atom is(are)

Reaction of iso-propylbenzene with $\mathrm{O}_2$ followed by the treatment with $\mathrm{H}_3 \mathrm{O}^{+}$forms phenol and a by-product $\mathbf{P}$. Reaction of $\mathbf{P}$ with 3 equivalents of $\mathrm{Cl}_2$ gives compound $\mathbf{Q}$. Treatment of $\mathbf{Q}$ with $\mathrm{Ca}(\mathrm{OH})_2$ produces compound $\mathbf{R}$ and calcium salt $\mathbf{S}$.
The correct statement(s) regarding $\mathbf{P}, \mathbf{Q}, \mathbf{R}$ and $\mathbf{S}$ is(are)

The option(s) in which at least three molecules follow Octet Rule is(are)

Consider the following volume-temperature $(\mathrm{V}-\mathrm{T})$ diagram for the expansion of 5 moles of an ideal monoatomic gas.
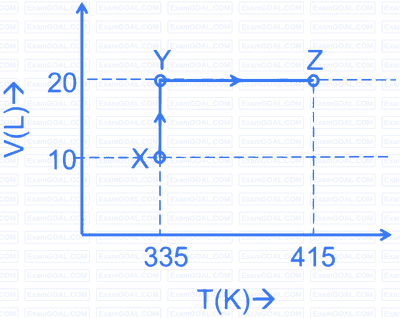
Considering only $\mathrm{P}-\mathrm{V}$ work is involved, the total change in enthalpy (in Joule) for the transformation of state in the sequence $\mathbf{X} \rightarrow \mathbf{Y} \rightarrow \mathbf{Z}$ is ____________.
[Use the given data: Molar heat capacity of the gas for the given temperature range, $\mathrm{C}_{\mathrm{V}, \mathrm{m}}=12 \mathrm{~J} \mathrm{~K}^{-1}$ $\mathrm{mol}^{-1}$ and gas constant, $\left.\mathrm{R}=8.3 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}\right]$
