1
JEE Advanced 2017 Paper 1 Offline
MCQ (More than One Correct Answer)
+4
-1

The IUPAC name(s) of the following compound is (are)


2
JEE Advanced 2017 Paper 1 Offline
MCQ (Single Correct Answer)
+3
-1

Columns 1, 2 and 3 contain starting materials, reaction conditions, and type of reactions, respectively.
| Column 1 | Column 2 | Column 3 | |||
|---|---|---|---|---|---|
| (I) | Toluene | (i) | NaOH/Br2 | (P) | Condensation |
| (II) | Acetophenone | (ii) | Br2/hv | (Q) | Carboxylation |
| (III) | Benzaldehyde | (iii) | (CH3CO)2O/ CH3COOK |
(R) | Substitution |
| (IV) | Phenol | (iv) | NaOH/CO2 | (S) | Haloform |
The only CORRECT combination in which the reaction proceeds through radical mechanism is :
3
JEE Advanced 2017 Paper 1 Offline
MCQ (Single Correct Answer)
+3
-1

Columns 1, 2 and 3 contain starting materials, reaction conditions, and type of reactions, respectively.
| Column 1 | Column 2 | Column 3 | |||
|---|---|---|---|---|---|
| (I) | Toluene | (i) | NaOH/Br2 | (P) | Condensation |
| (II) | Acetophenone | (ii) | Br2/hv | (Q) | Carboxylation |
| (III) | Benzaldehyde | (iii) | (CH3CO)2O/ CH3COOK |
(R) | Substitution |
| (IV) | Phenol | (iv) | NaOH/CO2 | (S) | Haloform |
The only CORRECT combination that gives two different carboxylic acids is :
4
JEE Advanced 2017 Paper 1 Offline
MCQ (Single Correct Answer)
+3
-0.75

The wave function, $${\psi _{n,1,{m_1}}}$$ is a mathematical function whose value depends upon spherical polar coordinates $$\left( {r,\theta ,\phi } \right)$$ of the electron and characterized by the quantum numbers $$n,1$$ and $${m_1}$$. Here $$r$$ is distance from nucleus, $$\theta $$ is colatitude and $$\phi $$ is azimuth. In the mathematical functions given in the table, $$Z$$ is atomic number and $${a_0}$$ is Bohr radius.
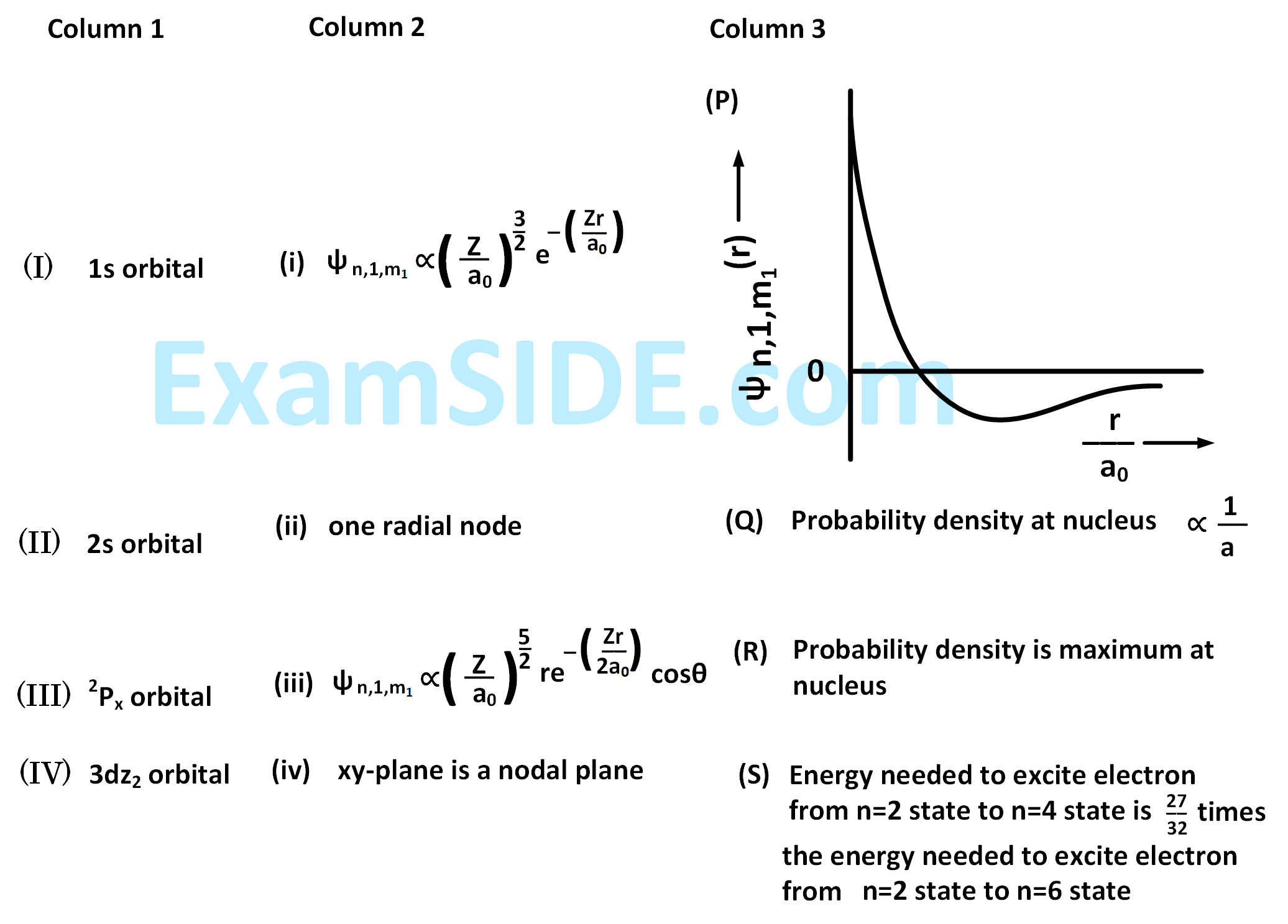

For $$H{e^ + }$$ ion, the only INCORRECT combination is
Paper analysis
Total Questions
Chemistry
18
Mathematics
18
Physics
18
More papers of JEE Advanced
JEE Advanced 2025 Paper 2 Online
JEE Advanced 2025 Paper 1 Online
JEE Advanced 2024 Paper 2 Online
JEE Advanced 2024 Paper 1 Online
JEE Advanced 2023 Paper 2 Online
JEE Advanced 2023 Paper 1 Online
JEE Advanced 2022 Paper 2 Online
JEE Advanced 2022 Paper 1 Online
JEE Advanced 2021 Paper 2 Online
JEE Advanced 2021 Paper 1 Online
JEE Advanced 2020 Paper 2 Offline
JEE Advanced 2020 Paper 1 Offline
JEE Advanced 2019 Paper 2 Offline
JEE Advanced 2019 Paper 1 Offline
JEE Advanced 2018 Paper 2 Offline
JEE Advanced 2018 Paper 1 Offline
JEE Advanced 2017 Paper 2 Offline
JEE Advanced 2017 Paper 1 Offline
JEE Advanced 2016 Paper 2 Offline
JEE Advanced 2016 Paper 1 Offline
JEE Advanced 2015 Paper 2 Offline
JEE Advanced 2015 Paper 1 Offline
JEE Advanced 2014 Paper 2 Offline
JEE Advanced 2014 Paper 1 Offline
JEE Advanced 2013 Paper 2 Offline
JEE Advanced 2013 Paper 1 Offline
IIT-JEE 2012 Paper 2 Offline
IIT-JEE 2012 Paper 1 Offline
IIT-JEE 2011 Paper 1 Offline
IIT-JEE 2011 Paper 2 Offline
IIT-JEE 2010 Paper 1 Offline
IIT-JEE 2010 Paper 2 Offline
IIT-JEE 2009 Paper 2 Offline
IIT-JEE 2009 Paper 1 Offline
IIT-JEE 2008 Paper 2 Offline
IIT-JEE 2008 Paper 1 Offline
IIT-JEE 2007
IIT-JEE 2007 Paper 2 Offline
IIT-JEE 2006
IIT-JEE 2006 Screening
IIT-JEE 2005 Screening
IIT-JEE 2005
IIT-JEE 2004 Screening
IIT-JEE 2004
IIT-JEE 2003
IIT-JEE 2003 Screening
IIT-JEE 2002
IIT-JEE 2002 Screening
IIT-JEE 2001 Screening
IIT-JEE 2001
IIT-JEE 2000 Screening
IIT-JEE 2000
IIT-JEE 1999 Screening
IIT-JEE 1999
IIT-JEE 1998 Screening
IIT-JEE 1998
IIT-JEE 1997
IIT-JEE 1996
IIT-JEE 1995 Screening
IIT-JEE 1995
IIT-JEE 1994
IIT-JEE 1993
IIT-JEE 1992
IIT-JEE 1991
IIT-JEE 1990
IIT-JEE 1989
IIT-JEE 1988
IIT-JEE 1987
IIT-JEE 1986
IIT-JEE 1985
IIT-JEE 1984
IIT-JEE 1983
IIT-JEE 1982
IIT-JEE 1981
IIT-JEE 1980
IIT-JEE 1979
IIT-JEE 1978
JEE Advanced
Papers
2020
2019
2018
2017
2016
1997
1996
1994
1993
1992
1991
1990
1989
1988
1987
1986
1985
1984
1983
1982
1981
1980
1979
1978