1
JEE Advanced 2017 Paper 1 Offline
Numerical
+3
-0

Among $${H_2},H{e_2}^ + ,L{i_2},$$ $$B{e_2},{B_2},{C_2},{N_2},O_2^ - $$ and $${F_2},$$ the number of diamagnetic species is
(Atomic numbers : $$H = 1,He = 2,$$ $$Li = 3,Be = 4,$$ $$B = 5,C = 6,$$ $$N = 7,$$ $$O = 8,F = 9$$)
(Atomic numbers : $$H = 1,He = 2,$$ $$Li = 3,Be = 4,$$ $$B = 5,C = 6,$$ $$N = 7,$$ $$O = 8,F = 9$$)
Your input ____
2
JEE Advanced 2017 Paper 1 Offline
MCQ (Single Correct Answer)
+3
-1

Columns 1, 2 and 3 contain starting materials, reaction conditions, and type of reactions, respectively.
| Column 1 | Column 2 | Column 3 | |||
|---|---|---|---|---|---|
| (I) | Toluene | (i) | NaOH/Br2 | (P) | Condensation |
| (II) | Acetophenone | (ii) | Br2/hv | (Q) | Carboxylation |
| (III) | Benzaldehyde | (iii) | (CH3CO)2O/ CH3COOK |
(R) | Substitution |
| (IV) | Phenol | (iv) | NaOH/CO2 | (S) | Haloform |
For the synthesis of benzoic acid, the only CORRECT combination is
3
JEE Advanced 2017 Paper 1 Offline
MCQ (More than One Correct Answer)
+4
-1

The IUPAC name(s) of the following compound is (are)


4
JEE Advanced 2017 Paper 1 Offline
MCQ (More than One Correct Answer)
+4
-1

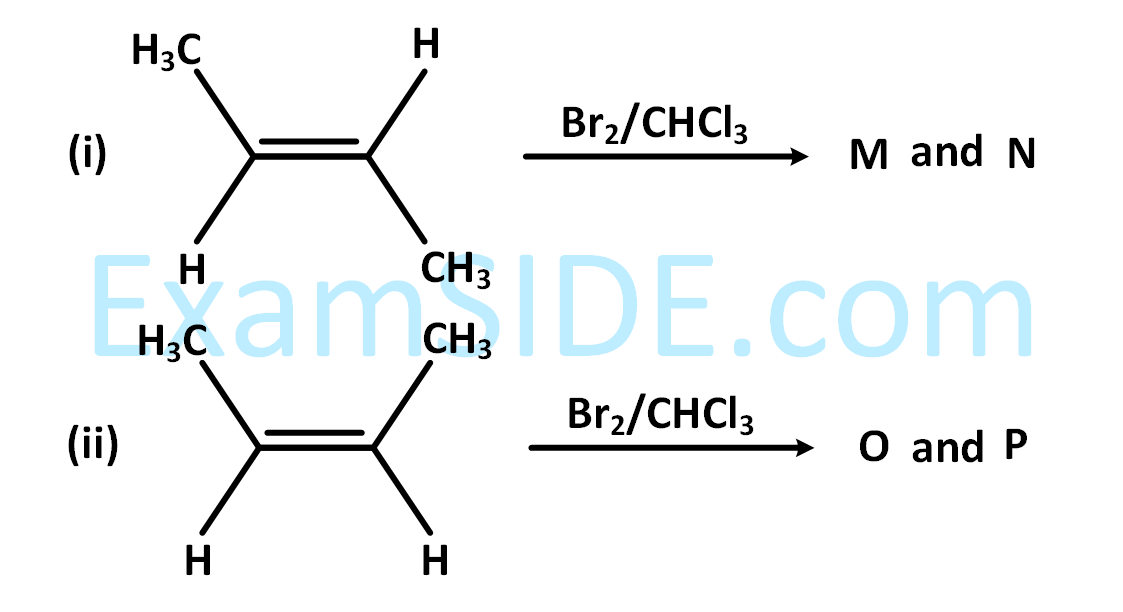
The correct statements(s) for the following addition reactions is (are)

Paper analysis
Total Questions
Chemistry
18
Mathematics
18
Physics
18
More papers of JEE Advanced
JEE Advanced 2025 Paper 2 Online
JEE Advanced 2025 Paper 1 Online
JEE Advanced 2024 Paper 2 Online
JEE Advanced 2024 Paper 1 Online
JEE Advanced 2023 Paper 2 Online
JEE Advanced 2023 Paper 1 Online
JEE Advanced 2022 Paper 2 Online
JEE Advanced 2022 Paper 1 Online
JEE Advanced 2021 Paper 2 Online
JEE Advanced 2021 Paper 1 Online
JEE Advanced 2020 Paper 2 Offline
JEE Advanced 2020 Paper 1 Offline
JEE Advanced 2019 Paper 2 Offline
JEE Advanced 2019 Paper 1 Offline
JEE Advanced 2018 Paper 2 Offline
JEE Advanced 2018 Paper 1 Offline
JEE Advanced 2017 Paper 2 Offline
JEE Advanced 2017 Paper 1 Offline
JEE Advanced 2016 Paper 2 Offline
JEE Advanced 2016 Paper 1 Offline
JEE Advanced 2015 Paper 2 Offline
JEE Advanced 2015 Paper 1 Offline
JEE Advanced 2014 Paper 2 Offline
JEE Advanced 2014 Paper 1 Offline
JEE Advanced 2013 Paper 2 Offline
JEE Advanced 2013 Paper 1 Offline
IIT-JEE 2012 Paper 2 Offline
IIT-JEE 2012 Paper 1 Offline
IIT-JEE 2011 Paper 1 Offline
IIT-JEE 2011 Paper 2 Offline
IIT-JEE 2010 Paper 2 Offline
IIT-JEE 2010 Paper 1 Offline
IIT-JEE 2009 Paper 2 Offline
IIT-JEE 2009 Paper 1 Offline
IIT-JEE 2008 Paper 2 Offline
IIT-JEE 2008 Paper 1 Offline
IIT-JEE 2007
IIT-JEE 2007 Paper 2 Offline
IIT-JEE 2006
IIT-JEE 2006 Screening
IIT-JEE 2005 Screening
IIT-JEE 2005
IIT-JEE 2004
IIT-JEE 2004 Screening
IIT-JEE 2003
IIT-JEE 2003 Screening
IIT-JEE 2002
IIT-JEE 2002 Screening
IIT-JEE 2001
IIT-JEE 2001 Screening
IIT-JEE 2000 Screening
IIT-JEE 2000
IIT-JEE 1999 Screening
IIT-JEE 1999
IIT-JEE 1998
IIT-JEE 1998 Screening
IIT-JEE 1997
IIT-JEE 1996
IIT-JEE 1995 Screening
IIT-JEE 1995
IIT-JEE 1994
IIT-JEE 1993
IIT-JEE 1992
IIT-JEE 1991
IIT-JEE 1990
IIT-JEE 1989
IIT-JEE 1988
IIT-JEE 1987
IIT-JEE 1986
IIT-JEE 1985
IIT-JEE 1984
IIT-JEE 1983
IIT-JEE 1982
IIT-JEE 1981
IIT-JEE 1980
IIT-JEE 1979
IIT-JEE 1978
JEE Advanced
Papers
2020
2019
2018
2017
2016
1997
1996
1994
1993
1992
1991
1990
1989
1988
1987
1986
1985
1984
1983
1982
1981
1980
1979
1978