1
JEE Advanced 2016 Paper 2 Offline
MCQ (Single Correct Answer)
+3
-1

The ends Q and R of two thin wires, PQ and RS, are soldered (joined) together. Initially each of the wires
has a length of 1 m at 10oC. Now the end P is maintained at 10oC, while the end S is heated and
maintained at 400oC. The system is thermally insulated from its surroundings. If the thermal conductivity
of wire PQ is twice that of the wire RS and the coefficient of linear thermal expansion of PQ is 1.2 $$ \times $$ 10-5 K-1 , the change in length of the wire PQ is
2
JEE Advanced 2016 Paper 2 Offline
MCQ (Single Correct Answer)
+3
-1

A gas is enclosed in a cylinder with a movable frictionless piston. Its initial thermodynamic state at
pressure Pi = 105 Pa and volume Vi = 10-3 m3 changes to a final state at Pf = $$\left( {{1 \over {32}}} \right) \times {10^5}\,Pa$$ and
Vf = 8 $$ \times $$ 10-3 m3 in an adiabatic quasi-static process, such that P3V5 = constant. Consider another
thermodynamic process that brings the system from the same initial state to the same final state in two
steps: an isobaric expansion at Pi followed by an isochoric (isovolumetric) process at volume Vf. The amount of heat supplied to the system in the two-step process is approximately
3
JEE Advanced 2016 Paper 1 Offline
MCQ (Single Correct Answer)
+3
-1

A water cooler of storage capacity 120 litres can cool water at a constant rate of P watts. In a closed circulation system (as shown schematically in the figure), the water from the cooler is used to cool an external device that generates constantly 3 kW of heat (thermal load).
The temperature of water fed into the device cannot exceed 30°C and the entire stored 120 litres of water is initially cooled to 10°C. The entire system is thermally insulated. The minimum value of P (in watts) for which the device can be operated for 3 hours is :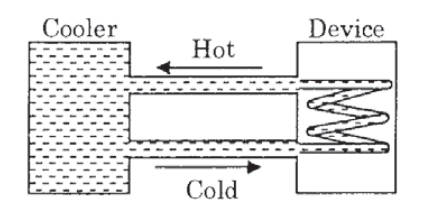
(Specific heat of water is 4.2 kJ kg−1 K−1 and the density of water is 1000 kg m−3)
The temperature of water fed into the device cannot exceed 30°C and the entire stored 120 litres of water is initially cooled to 10°C. The entire system is thermally insulated. The minimum value of P (in watts) for which the device can be operated for 3 hours is :

(Specific heat of water is 4.2 kJ kg−1 K−1 and the density of water is 1000 kg m−3)
4
JEE Advanced 2014 Paper 2 Offline
MCQ (Single Correct Answer)
+3
-1
Parallel rays of light of intensity $$I$$ = 912 Wm–2 are incident on a spherical black body kept in surroundings
of temperature 300 K. Take Stefan-Boltzmann constant $$\sigma $$ = 5.7 $$ \times $$ 10–8 Wm–2K–4 and assume that the energy
exchange with the surroundings is only through radiation. The final steady state temperature of the black
body is close to
Questions Asked from Heat and Thermodynamics (MCQ (Single Correct Answer))
Number in Brackets after Paper Indicates No. of Questions
JEE Advanced 2024 Paper 1 Online (1)
JEE Advanced 2023 Paper 2 Online (1)
JEE Advanced 2023 Paper 1 Online (2)
JEE Advanced 2022 Paper 1 Online (1)
JEE Advanced 2021 Paper 2 Online (2)
JEE Advanced 2021 Paper 1 Online (1)
JEE Advanced 2019 Paper 2 Offline (2)
JEE Advanced 2018 Paper 2 Offline (1)
JEE Advanced 2017 Paper 1 Offline (3)
JEE Advanced 2016 Paper 2 Offline (2)
JEE Advanced 2016 Paper 1 Offline (1)
JEE Advanced 2014 Paper 2 Offline (3)
JEE Advanced 2013 Paper 2 Offline (1)
JEE Advanced 2013 Paper 1 Offline (2)
IIT-JEE 2012 Paper 2 Offline (1)
IIT-JEE 2012 Paper 1 Offline (2)
IIT-JEE 2011 Paper 1 Offline (1)
IIT-JEE 2011 Paper 2 Offline (1)
IIT-JEE 2010 Paper 1 Offline (1)
IIT-JEE 2009 Paper 2 Offline (1)
IIT-JEE 2008 Paper 2 Offline (1)
IIT-JEE 2008 Paper 1 Offline (1)
IIT-JEE 2007 Paper 2 Offline (1)
JEE Advanced Subjects
Physics
Mechanics
Units & Measurements Motion Laws of Motion Work Power & Energy Impulse & Momentum Rotational Motion Properties of Matter Heat and Thermodynamics Simple Harmonic Motion Waves Gravitation
Electricity
Electrostatics Current Electricity Capacitor Magnetism Electromagnetic Induction Alternating Current Electromagnetic Waves
Optics
Modern Physics
Chemistry
Physical Chemistry
Some Basic Concepts of Chemistry Structure of Atom Redox Reactions Gaseous State Chemical Equilibrium Ionic Equilibrium Solutions Thermodynamics Chemical Kinetics and Nuclear Chemistry Electrochemistry Solid State Surface Chemistry
Inorganic Chemistry
Periodic Table & Periodicity Chemical Bonding & Molecular Structure Isolation of Elements Hydrogen s-Block Elements p-Block Elements d and f Block Elements Coordination Compounds Salt Analysis
Organic Chemistry
Mathematics
Algebra
Quadratic Equation and Inequalities Sequences and Series Mathematical Induction and Binomial Theorem Matrices and Determinants Permutations and Combinations Probability Vector Algebra 3D Geometry Statistics Complex Numbers
Trigonometry
Coordinate Geometry
Calculus