Thermodynamics · Chemistry · JEE Advanced
Numerical
Considering ideal gas behavior, the expansion work done (in kJ) when 144 g of water is electrolyzed completely under constant pressure at 300 K is ______.
Use: Universal gas constant (R) = 8.3 J K−1 mol−1; Atomic mass (in amu): H = 1, O = 16
Consider the following volume-temperature $(\mathrm{V}-\mathrm{T})$ diagram for the expansion of 5 moles of an ideal monoatomic gas.
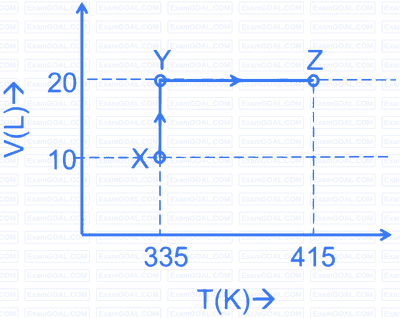
Considering only $\mathrm{P}-\mathrm{V}$ work is involved, the total change in enthalpy (in Joule) for the transformation of state in the sequence $\mathbf{X} \rightarrow \mathbf{Y} \rightarrow \mathbf{Z}$ is ____________.
[Use the given data: Molar heat capacity of the gas for the given temperature range, $\mathrm{C}_{\mathrm{V}, \mathrm{m}}=12 \mathrm{~J} \mathrm{~K}^{-1}$ $\mathrm{mol}^{-1}$ and gas constant, $\left.\mathrm{R}=8.3 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}\right]$
[Use : $\ln 2=0.69$
Given : $S_\beta-S_\alpha=0$ at $0 \mathrm{~K}$ ]
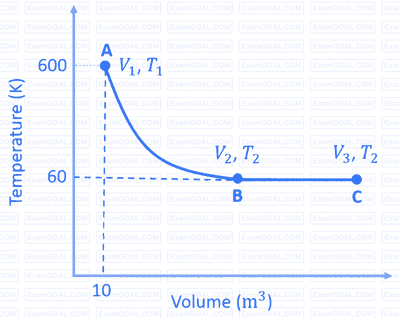
$\mathrm{A} \rightarrow \mathrm{B}$ is an adiabatic process. If the total heat absorbed in the entire process $(\mathrm{A} \rightarrow \mathrm{B}$ and $\mathrm{B} \rightarrow \mathrm{C})$ is $\mathrm{R} T_2 \ln 10$, the value of $2 \log V_3$ is _______.
[Use, molar heat capacity of the gas at constant pressure, $C_{\mathrm{p}, \mathrm{m}}=\frac{5}{2} \mathrm{R}$ ]

If $\mathrm{T}_1=2 \mathrm{~T}_2$ and $\left(\Delta \mathrm{G}_2^{\Theta}-\Delta \mathrm{G}_1^{\Theta}\right)=\mathrm{RT}_2 \ln \mathrm{x}$, then the value of $\mathrm{x}$ is _______.
$\left[\Delta \mathrm{G}_1^{\Theta}\right.$ and $\Delta \mathrm{G}_2^{\Theta}$ are standard Gibb's free energy change for the reaction at temperatures $\mathrm{T}_1$ and $\mathrm{T}_2$, respectively.]
$2 \mathrm{~mol} \,\mathrm{of}\, \mathrm{Hg}(\mathrm{g})$ is combusted in a fixed volume bomb calorimeter with excess of $\mathrm{O}_{2}$ at $298 \mathrm{~K}$ and 1 atm into $\mathrm{HgO}(s)$. During the reaction, temperature increases from $298.0 \mathrm{~K}$ to $312.8 \mathrm{~K}$. If heat capacity of the bomb calorimeter and enthalpy of formation of $\mathrm{Hg}(g)$ are $20.00 \mathrm{~kJ} \mathrm{~K}^{-1}$ and $61.32 \mathrm{~kJ}$ $\mathrm{mol}^{-1}$ at $298 \mathrm{~K}$, respectively, the calculated standard molar enthalpy of formation of $\mathrm{HgO}(s)$ at 298 $\mathrm{K}$ is $\mathrm{X}\, \mathrm{kJ}\, \mathrm{mol}^{-1}$. The value of $|\mathrm{X}|$ is _________ .
[Given: Gas constant $\mathrm{R}=8.3 \mathrm{~J} \mathrm{~K}^{-1} \mathrm{~mol}^{-1}$ ]
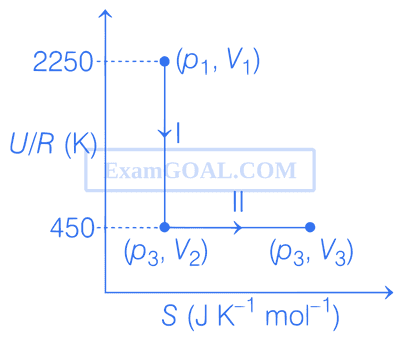
(U : internal energy, S : entropy, p : pressure, V : volume, R : gas constant)
(Given : molar heat capacity at constant volume, CV,m of the gas is $${5 \over 2}$$R)
At $$298K:{\Delta _f}H^\circ [Sn{O_2}(s)] = - 581.0$$ mol-1,
$$\eqalign{ & {\Delta _f}H^\circ [(C{O_2})(g)] = - 394.0\,kJ\,mol{ ^{-1}} \cr & S^\circ [Sn{O_2}(s)] = 56.0J\,{K^{ - 1}}mo{l^{ - 1}} \cr & S^\circ [Sn(s)] = 52.0\,J\,K{ ^{-1}}mo{l^{ - 1}} \cr & S^\circ [C(s)] = 6.0\,J\,{K^{ - 1}}mo{l^{ - 1}} \cr & S^\circ [C{O_2}(g)] = 210.0\,J\,{K^{ - 1}}mo{l^{ - 1}} \cr} $$
Assume that, the enthalpies and the entropies are temperature independent.
$${P_{H2}}$$ is the minimum partial pressure of $${H_2}$$ (in bar) needed to prevent the oxidation at $$1250$$ $$K.$$ The value of $$\ln \left( {{P_{H2}}} \right)$$ is ________.
Given: total pressure $$=1$$ bar, $$R$$ (universal gas constant ) $$=$$ $$8J{K^{ - 1}}\,\,mo{l^{ - 1}},$$ $$\ln \left( {10} \right) = 2.3.\,$$ $$Cu(s)$$ and $$C{u_2}O\left( s \right)$$ are naturally immiscible.
At $$1250$$ $$K:2Cu(s)$$ $$ + {\raise0.5ex\hbox{$\scriptstyle 1$} \kern-0.1em/\kern-0.15em \lower0.25ex\hbox{$\scriptstyle 2$}}{O_2}\left( g \right) \to C{u_2}O\left( s \right);$$ $$\Delta {G^ \circ } = - 78,000J\,mo{l^{ - 1}}$$
$${H_2}\left( g \right) + {\raise0.5ex\hbox{$\scriptstyle 1$} \kern-0.1em/\kern-0.15em \lower0.25ex\hbox{$\scriptstyle 2$}}{O_2}\left( g \right) \to {H_2}O\left( g \right);$$
$$\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,$$ $$\Delta {G^ \circ } = - 1,78,000J\,mo{l^{ - 1}};$$ ($$G$$ is the Gibbs energy)
One mole of an ideal gas is taken from $\mathbf{a}$ to $\mathbf{b}$ along two paths denoted by the solid and the dashed lines as shown in the graph below. If the work done along the solid line path is $W_{\text {s }}$ and that dotted line path is $W_{\mathrm{d}}$, then the integer closest to the ratio $W_{\mathrm{d}} / W_{\mathrm{s}}$ is

In a constant volume calorimeter, 3.5 g of a gas with molecular weight 28 was burnt in excess oxygen at 298.0 K. The temperature of the calorimeter was found to increase from 298.0 K to 298.45 K due to the combustion process. Given that the heat capacity of the calorimeter is 2.5 kJ K$$^{-1}$$, the numerical value for the enthalpy of combustion of the gas in kJ mol$$^{-1}$$ is ____________.
MCQ (More than One Correct Answer)
$[\mathrm{R}=$ gas constant, $\mathrm{F}=$ Faraday constant, $\mathrm{T}=$ Temperature $]$
(p : pressure, V : volume, T : temperature, H : enthalpy, S : entropy)
$$w = - \int {dV{p_{ext}}} $$
For a system undergoing a particular process, the work done is
$$w = - \int {dV\left( {{{RT} \over {V - b}} - {a \over {{V^2}}}} \right)} $$
This equation is applicable to a

If $${T_2} > {T_1},$$ the correct statement(s) is (are) (Assume $$\Delta {H^ \circ }$$ and $$\Delta {S^ \circ }$$ are independent of temperature and ratio of $$lnK$$ at $${T_1}$$ to $$lnK$$ at $${T_2}$$ is greater than $${{{T_2}} \over {{T_1}}}.$$ Here $$H,$$ $$S,G$$ and $$K$$ are enthalpy, entropy, Gibbs energy and equilibrium constant, respectively.)

The correct option(s) is (are)
The reversible expansion of an ideal gas under adiabatic and isothermal conditions is shown in the figure. Which of the following statement(s) is(are) correct?

For an ideal gas, consider only P-V work in going from an initial state X to the final state Z. The final state Z can be reached by either of the two paths shown in the figure. Which of the following choice(s) is(are) correct? (Take $$\Delta$$S as change in entropy and W as work done)

Among the following, the state function(s) is(are)
MCQ (Single Correct Answer)
$${\Delta _f}{G^0}$$ [$$C$$(graphite)] $$ = 0kJmo{l^{ - 1}}$$
$${\Delta _f}{G^0}$$ [$$C$$(diamond)] $$ = 2.9kJmo{l^{ - 1}}$$
The standard state means that the pressure should be $$1$$ bar, and substance should be pure at a given temperature. The conversion of graphite [$$C$$(graphite)] to diamond [$$C$$(diamond)] reduces its volume by $$2 \times {10^{ - 6}}\,{m^3}\,mo{l^{ - 1}}$$ If $$C$$(graphite) is converted to $$C$$(diamond) isothermally at $$T=298$$ $$K,$$ the pressure at which $$C$$(graphite) is in equilibrium with $$C$$(diamond), is
[Useful information : $$1$$ $$J=1$$ $$kg\,{m^2}{s^{ - 2}};1\,Pa = 1\,kg\,{m^{ - 1}}{s^{ - 2}};$$ $$1$$ bar $$ = {10^5}$$ $$Pa$$]
Column I
(A) Freezing water at 273 K and 1 atm
(B) Expansion of 1 mol of an ideal gas into a vacuum under isolated conditions.
(C) Mixing of equal volumes of two ideal gases at constant temperature and pressure in an isolated container.
(D) Reversible heating of H2(g) at 1 atm from 300K to 600K, followed by reversible cooling to 300K at 1 atm
Column II
(p) q = 0
(q) w = 0
(r) $$\Delta S_{sys}$$ < 0
(s) $$\Delta U$$ = 0
(t) $$\Delta G$$ = 0
H2O(l) $$\to$$ H2O(g)
at T = 100oC and 1 atmosphere pressure, the correct choice is
The succeeding operations that enable this transformation of states are
The pair of isochoric processes among the transformation of states is
Using the data provided, calculate the multiple bond energy (kJ mol$$-$$1) of a C=C bond in C2H2. That energy is (take the bond energy of C-H bond as 350 kJ mol$$-$$1).
$$\matrix{ \hfill {2C(s) + {H_2}(g) \to {C_2}{H_2}} & \hfill {\Delta H = 225\,kJ\,mo{l^{ - 1}}} \cr \hfill {2C(s) \to 2C(g)} & \hfill {\Delta H = 1410\,kJ\,mo{l^{ - 1}}} \cr \hfill {{H_2}(g) \to 2H(g)} & \hfill {\Delta H = 330\,kJ\,mo{l^{ - 1}}} \cr } $$
Column I
(A) CO2(s) $$\to$$ CO2(g)
(B) CaCO3(s) $$\to$$ CaO(s) + CO2(g)
(C) 2H $$\to$$ H2(g)
(D) P(white, solid) $$\to$$ P(red, solid)
Column II
(p) phase transition
(q) allotropic change
(r) $$\Delta H$$ is positive
(s) $$\Delta S$$ is positive
(t) $$\Delta S$$ is negative
Statement 1 : There is a natural asymmetry between converting work to heat and converting heat to work.
Statement 2 : No process is possible in which the sole result is the absorption of heat from a reservoir and its complete conversion into work.