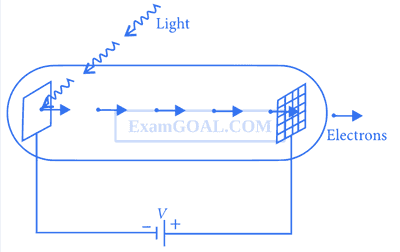
Dual Nature of Radiation · Physics · JEE Advanced
Numerical
[Given: $h c=1240 \mathrm{eV}-\mathrm{nm}$ and $R h c=13.6 \mathrm{eV}$, where $R$ is the Rydberg constant, $h$ is the Planck's constant and $c$ is the speed of light in vacuum]
The work functions of silver and sodium are 4.6 and 2.3 eV, respectively. The ratio of the slope of the stopping potential versus frequency plot for silver to that of sodium is ___________.
A proton is fired from very far away towards a nucleus with charge Q = 120e, where e is the electronic charge. It makes a closest approach of 10 fm to the nucleus. The de Broglie wavelength (in units of fm) of the proton at its start is ____________. (Take the proton mass, $${m_p} = (5 \times 3) \times {10^{ - 27}}$$ kg; $$h/e = 4.2 \times {10^{ - 15}}$$ J.s/C; $${1 \over {4\pi {\varepsilon _0}}} = 9 \times {10^9}$$ m/F; 1 fm = 1015 m.)
The activity of a freshly prepared radioactive sample is 1010 disintegrations per second, whose mean life is 109 s. The mass of an atom of this radioisotope is 10$$-$$25 kg. The mass (in mg) of the radioactive sample is _________.
A silver sphere of radius 1 cm and work function 4.7 eV is suspended from an insulating thread in free-space. It is under continuous illumination of 200 nm wavelength light. As photoelectrons are emitted, the sphere gets charged and acquires a potential. The maximum number of photoelectrons emitted from the spheres is A $$\times$$ 10Z (where 1 < A < 10). The value of Z is _____________.
An $$\alpha$$-particle and a proton are accelerated from the rest by a potential difference of 100 V. After this, their de Broglie wavelengths are $$\lambda$$$$\alpha$$ and $$\lambda$$p, respectively. The ratio $${{{\lambda _p}} \over {{\lambda _\alpha }}}$$, to the nearest integer, is _____________.
MCQ (Single Correct Answer)
When light of a given wavelength is incident on a metallic surface, the minimum potential needed to stop the emitted photoelectrons is $6.0 \mathrm{~V}$. This potential drops to $0.6 \mathrm{~V}$ if another source with wavelength four times that of the first one and intensity half of the first one is used. What are the wavelength of the first source and the work function of the metal, respectively? [Take $\frac{h c}{e}=1.24 \times$ $10^{-6} \mathrm{JmC}^{-1}$.]
| $$\lambda \left( {\mu m} \right)$$ | V0(Volt) |
|---|---|
| 0.3 | 2.0 |
| 0.4 | 1.0 |
| 0.5 | 0.4 |
Given that c = 3 $$ \times $$ 108 ms-1 and e = 1.6 $$ \times $$ 10-19 C, Planck's constant (in units of J-s) found from such an experiment is) :
A metal surface is illuminated by light of two different wavelengths 248 nm and 310 nm. The maximum speeds of the photoelectrons corresponding to these wavelengths are u1 and u2, respectively. If the ratio u1 : u2 = 2 : 1 and hc = 1240 eV nm, the work function of the metal is nearly
Photoelectric effect experiments are performed using three different metal plates p, q and r having work functions $$\phi_p=2.0~\mathrm{eV}$$, $$\phi_q=2.5~\mathrm{eV}$$ and $$\phi_r=3.0~\mathrm{eV}$$, respecticely. A light beam containing wavelengths of 550 nm, 450 nm and 350 nm with equal intensities illuminates each of the plates. The correct I-V graph for the experiment is (Take hc = 1240 eV nm)
The allowed energy for the particle for a particular value of $$n$$ is proportional to
If the mass of the particle is $$m=1.0\times10^{-30}$$ kg and $$a=6.6$$ nm, the energy of the particle in its ground state is closest to
Which one of the following statements is WRONG in the context of X-rays generated from a X-ray tube?
Electrons with de-Broglie wavelength $$\lambda$$ fall on the target in an X-ray tube. The cut-off wavelength of the emitted X-rays is
MCQ (More than One Correct Answer)