Chemical Equilibrium · Chemistry · JEE Advanced
Numerical
1
The plot of $\log k_f$ versus $1 / T$ for a reversible reaction $\mathrm{A}(\mathrm{g}) \rightleftharpoons \mathrm{P}(\mathrm{g})$ is shown.
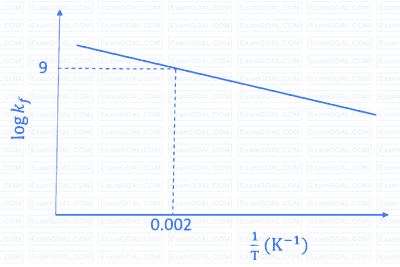
Pre-exponential factors for the forward and backward reactions are $10^{15} \mathrm{~s}^{-1}$ and $10^{11} \mathrm{~s}^{-1}$, respectively. If the value of $\log K$ for the reaction at $500 \mathrm{~K}$ is 6 , the value of $\left|\log k_b\right|$ at $250 \mathrm{~K}$ is ______.
$$ \begin{aligned} & {[K=\text { equilibrium constant of the reaction }} \\\\ & k_f=\text { rate constant of forward reaction } \\\\ & \left.k_b=\text { rate constant of backward reaction }\right] \end{aligned} $$

Pre-exponential factors for the forward and backward reactions are $10^{15} \mathrm{~s}^{-1}$ and $10^{11} \mathrm{~s}^{-1}$, respectively. If the value of $\log K$ for the reaction at $500 \mathrm{~K}$ is 6 , the value of $\left|\log k_b\right|$ at $250 \mathrm{~K}$ is ______.
$$ \begin{aligned} & {[K=\text { equilibrium constant of the reaction }} \\\\ & k_f=\text { rate constant of forward reaction } \\\\ & \left.k_b=\text { rate constant of backward reaction }\right] \end{aligned} $$
JEE Advanced 2023 Paper 1 Online
2
Consider the reaction,
A $$\rightleftharpoons $$ B
at 1000 K. At time t', the temperature of the system was increased to 2000 K and the system was allowed to reach equilibrium. Throughout this experiment the partial pressure of A was maintained at 1 bar. Given, below is the plot of the partial pressure of B with time. What is the ratio of the standard Gibbs energy of the reaction at 1000 K to that at 2000 K?

A $$\rightleftharpoons $$ B
at 1000 K. At time t', the temperature of the system was increased to 2000 K and the system was allowed to reach equilibrium. Throughout this experiment the partial pressure of A was maintained at 1 bar. Given, below is the plot of the partial pressure of B with time. What is the ratio of the standard Gibbs energy of the reaction at 1000 K to that at 2000 K?

JEE Advanced 2020 Paper 1 Offline
3
For the following reaction, the equilibrium constant Kc at 298 K is 1.6 $$ \times $$ 1017.
Fe2+(aq) + S2-(aq) ⇌ FeS(s)
When equal volumes of
0.06 M Fe2+(aq) and 0.2 M S2$$ - $$(aq)
solutions are mixed, the equilibrium concentration of Fe2+(aq) is found by Y $$ \times $$ 10$$ - $$17 M. The value of Y is .................
Fe2+(aq) + S2-(aq) ⇌ FeS(s)
When equal volumes of
0.06 M Fe2+(aq) and 0.2 M S2$$ - $$(aq)
solutions are mixed, the equilibrium concentration of Fe2+(aq) is found by Y $$ \times $$ 10$$ - $$17 M. The value of Y is .................
JEE Advanced 2019 Paper 1 Offline
4
A closed tank has two compartments $$A$$ and $$B,$$ both filled with oxygen (assumed to be ideal gas). The partition separating the two compartments is fixed and is a perfect heat insulator (Figure $$1.$$). If the old partition is replaced by a new partition which can slide and conduct heat but does NOT allow the gas to leak across (Figure $$2$$), the volume (in $${m^3}$$) of the compartment A after the system attains equilibrium is ______________.


JEE Advanced 2018 Paper 1 Offline
MCQ (Single Correct Answer)
1
Paragraph
Thermal decomposition of gaseous X2 to gaseous X at 298 K takes place according to the following equations:
X2 (g) $$\leftrightharpoons$$ 2X (g)
The standard reaction Gibbs energy, $$\Delta _rG^o$$, of this reaction is positive. At the start of the reaction, there is one mole of X2 and no X. As the reaction proceeds, the number of moles of X formed is given by $$\beta$$. Thus, $$\beta _{equilibrium}$$ is the number of moles of X formed at equilibrium. The reaction is carried out at a constant total pressure of 2 bar. Consider the gases to behave ideally. (Given R = 0.083 L bar K-1 mol-1)
Question
The equilibrium constant Kp for this reaction at 298 K, in terms of $$\beta _{equilibrium}$$, is
Thermal decomposition of gaseous X2 to gaseous X at 298 K takes place according to the following equations:
X2 (g) $$\leftrightharpoons$$ 2X (g)
The standard reaction Gibbs energy, $$\Delta _rG^o$$, of this reaction is positive. At the start of the reaction, there is one mole of X2 and no X. As the reaction proceeds, the number of moles of X formed is given by $$\beta$$. Thus, $$\beta _{equilibrium}$$ is the number of moles of X formed at equilibrium. The reaction is carried out at a constant total pressure of 2 bar. Consider the gases to behave ideally. (Given R = 0.083 L bar K-1 mol-1)
Question
The equilibrium constant Kp for this reaction at 298 K, in terms of $$\beta _{equilibrium}$$, is
JEE Advanced 2016 Paper 2 Offline
2
Paragraph
Thermal decomposition of gaseous X2 to gaseous X at 298 K takes place according to the following equations:
X2 (g) $$\leftrightharpoons$$ 2X (g)
The standard reaction Gibbs energy, $$\Delta _rG^o$$, of this reaction is positive. At the start of the reaction, there is one mole of X2 and no X. As the reaction proceeds, the number of moles of X formed is given by $$\beta$$. Thus, $$\beta _{equilibrium}$$ is the number of moles of X formed at equilibrium. The reaction is carried out at a constant total pressure of 2 bar. Consider the gases to behave ideally. (Given R = 0.083 L bar K-1 mol-1)
Question
The INCORRECT statement among the following for this reaction, is
Thermal decomposition of gaseous X2 to gaseous X at 298 K takes place according to the following equations:
X2 (g) $$\leftrightharpoons$$ 2X (g)
The standard reaction Gibbs energy, $$\Delta _rG^o$$, of this reaction is positive. At the start of the reaction, there is one mole of X2 and no X. As the reaction proceeds, the number of moles of X formed is given by $$\beta$$. Thus, $$\beta _{equilibrium}$$ is the number of moles of X formed at equilibrium. The reaction is carried out at a constant total pressure of 2 bar. Consider the gases to behave ideally. (Given R = 0.083 L bar K-1 mol-1)
Question
The INCORRECT statement among the following for this reaction, is
JEE Advanced 2016 Paper 2 Offline
3
Statement 1 : For every chemical reaction at equilibrium, standard Gibbs energy of reaction is zero.
and
Statement 2 : At constant temperature and pressure, chemical reactions are spontaneous in the direction of decreasing Gibbs energy.
IIT-JEE 2008 Paper 1 Offline
MCQ (More than One Correct Answer)
1
The thermal dissociation equilibrium of CaCO3(s) is studied under different conditions
CaCO3(s) $$\leftrightharpoons$$ CaO(s) + CO2(g).
For this equilibrium, the correct statement(s) is (are)
CaCO3(s) $$\leftrightharpoons$$ CaO(s) + CO2(g).
For this equilibrium, the correct statement(s) is (are)
JEE Advanced 2013 Paper 2 Offline
2
The equilibrium
$$2C{u^+} \to Cu^\circ + C{u^{2+}}$$
In aqueous medium at 25$$^\circ$$C shifts towards the left in the presence of
IIT-JEE 2011 Paper 2 Offline