Compounds Containing Nitrogen · Chemistry · JEE Advanced
MCQ (More than One Correct Answer)
For the reaction sequence given below, the correct statement(s) is(are):

The option(s) with correct sequence of reagents for the conversion of $\mathbf{P}$ to $\mathbf{Q}$ is(are)

Considering the following reaction sequence,

the correct option(s) is(are)
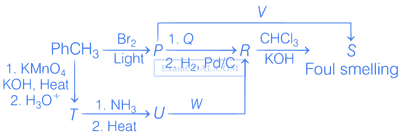

In the following reactions, the major product W is

The major product of the reaction is


MCQ (Single Correct Answer)
The major products obtained from the reactions in List-II are the reactants for the named reactions mentioned in List-I. Match each entry in List-I with the appropriate entry in List-II and choose the correct option.
| List–I | List–II |
|---|---|
| (P) Stephen reaction | (1) $$ \text { Toluene } \xrightarrow{\begin{array}{l} \text { (i) } \mathrm{CrO}_2 \mathrm{Cl}_2 / \mathrm{CS}_2 \\ \text { (ii) } \mathrm{H}_3 \mathrm{O}^{+} \end{array}} $$ |
| (Q) Sandmeyer reaction | (2) $$ \text { Benzoic acid } \xrightarrow{\substack{\text { (i) } \mathrm{PCl}_5 \\ \text { (ii) } \mathrm{NH}_3 \\ \text { (iii) } \mathrm{P}_4 \mathrm{O}_{10}, \Delta}} $$ |
| (R) Hoffmann bromamide degradation reaction | (3) $$ \text { Nitrobenzene } \xrightarrow{\begin{array}{l} \text { (i) } \mathrm{Fe}, \mathrm{HCl} \\ \text { (ii) } \mathrm{HCl}, \mathrm{NaNO}_2 \\ (273-278 \mathrm{~K}), \mathrm{H}_2 \mathrm{O} \end{array}} $$ |
| (S) Cannizzaro reaction | (4) $$ \text { Toluene } \xrightarrow{\begin{array}{ll} \text { (i) } \mathrm{Cl}_2 / \mathrm{h\nu}, \mathrm{H}_2 \mathrm{O} \\ \text { (ii) Tollen's reagent } \\ \text { (iii) } \mathrm{SO}_2 \mathrm{Cl}_2 \\ \text { (iv) } \mathrm{NH}_3 \end{array}} $$ |
| (5) $$ \text { Aniline } \xrightarrow{\begin{array}{l} \text { (i) }\left(\mathrm{CH}_3 \mathrm{CO}\right)_2 \mathrm{O}, \text { Pyridine } \\ \text { (ii) } \mathrm{HNO}_3, \mathrm{H}_2 \mathrm{SO}_4, 288 \mathrm{~K} \\ \text { (iii) aq. } \mathrm{NaOH} \end{array}} $$ |
List-I contains various reaction sequences and List-II contains different phenolic compounds. Match each entry in List-I with the appropriate entry in List-II and choose the correct option.
| List-I | List-II |
|---|---|

|

|

|

|

|

|

|

|

|
Match the compounds in LIST-I with the observations in LIST-II, and choose the correct option.
| List-I | List-II |
|---|---|
| (I) Aniline |
(P) Sodium fusion extract of the compound on boiling with $\mathrm{FeSO}_{4}$, followed by acidification with conc. $\mathrm{H}_{2} \mathrm{SO}_{4}$, gives Prussian blue color. |
| (II) $o$-Cresol | (Q) Sodium fusion extract of the compound on treatment with sodium nitroprusside gives blood red color. |
| (III) Cysteine |
(R) Addition of the compound to a saturated solution of $\mathrm{NaHCO}_{3}$ results in effervescence. |
| (IV) Caprolactam |
(S) The compound reacts with bromine water to give a white precipitate. |
| (T) Treating the compound with neutral $\mathrm{FeCl}_{3}$ solution produces violet color. |


The compound R is
The compound T is
Match the four starting materials (P, Q, R, S) given in List I with the corresponding reaction schemes (I, II, III, IV) provided in List II and select the correct answer using the code given below the lists.

The major product of the following reaction is

Amongst the compounds given, the one that would from a brilliant coloured dye on treatment with NaNO2 in dil. HCl followed by addition to an alkaline solution of $$\beta$$-naphthlol is
In the reaction,

The structure of the product T is :
| Column I | Column II |
|---|---|
(A) 
|
(P) Racemic mixture |
(B) 
|
(Q) Addition reaction |
(C) 
|
(R) Substitution reaction |
(D) 
|
(S) Coupling reaction |
Match each of the compounds in Column I with its characteristic reaction(s) in Column II.
| Column I | Column II | ||
|---|---|---|---|
| (A) | $$C{H_3}C{H_2}C{H_2}CN$$ | (P) | Reduction with $$Pd - C/{H_2}$$ |
| (B) | $$C{H_3}C{H_2}OCOC{H_3}$$ | (Q) | Reduction with $$SnC{l_2}/HCl$$ |
| (C) | $$C{H_3} - CH = CH - C{H_2}OH$$ | (R) | Development of foul smell on treatment with chloroform and alcoholic KOH |
| (D) | $$C{H_3}C{H_2}C{H_2}C{H_2}N{H_2}$$ | (S) | Reduction with diisobutylaluminium hydride (DIBAL-H) |
| (T) | Alkaline hydrolysis |
Statement 1 : Aniline on reaction with NaNO$$_2$$/HCl at 0$$^\circ$$C followed by coupling with $$\beta$$-naphthol gives a dark blue coloured precipitate.
Statement 2 : The colour of the compound formed in the reaction of aniline with NaNO$$_2$$/HCl at 0$$^\circ$$C followed by coupling with $$\beta$$-naphthol is due to the extended conjugation.
Match the compounds in Column I with their characteristic test(s)/reaction(s) given in Column II. Indicate your answer by darkening the appropriate bubbles of the 4 $$\times$$ 4 matrix given in the ORS.
| Column I | Column II | ||
|---|---|---|---|
| (A) |  |
(P) | sodium fusion extract of the compound gives Prussian blue colour with FeSO$$_4$$. |
| (B) |  |
(Q) | gives positive FeCl$$_3$$ test. |
| (C) |  |
(R) | gives white precipitate with AgNO$$_3$$. |
| (D) |  |
(S) | reacts with aldehydes to form the corresponding hydrazone derivative. |
Numerical
[Use : Molar mass (in $\left.\mathrm{g} \mathrm{mol}^{-1}\right)$ : $\mathrm{H}=1, \mathrm{C}=12, \mathrm{~N}=14, \mathrm{O}=16, \mathrm{Br}=80, \mathrm{Cl}=35.5$
Atoms other than $\mathrm{C}$ and $\mathrm{H}$ are considered as heteroatoms]
[Use : Molar mass (in $\mathrm{g} \mathrm{mol}^{-1}$ ): $\mathrm{H}=1, \mathrm{C}=12, \mathrm{~N}=14, \mathrm{O}=16, \mathrm{Br}=80, \mathrm{Cl}=35.5$
Atoms other than $\mathrm{C}$ and $\mathrm{H}$ are considered as heteroatoms]

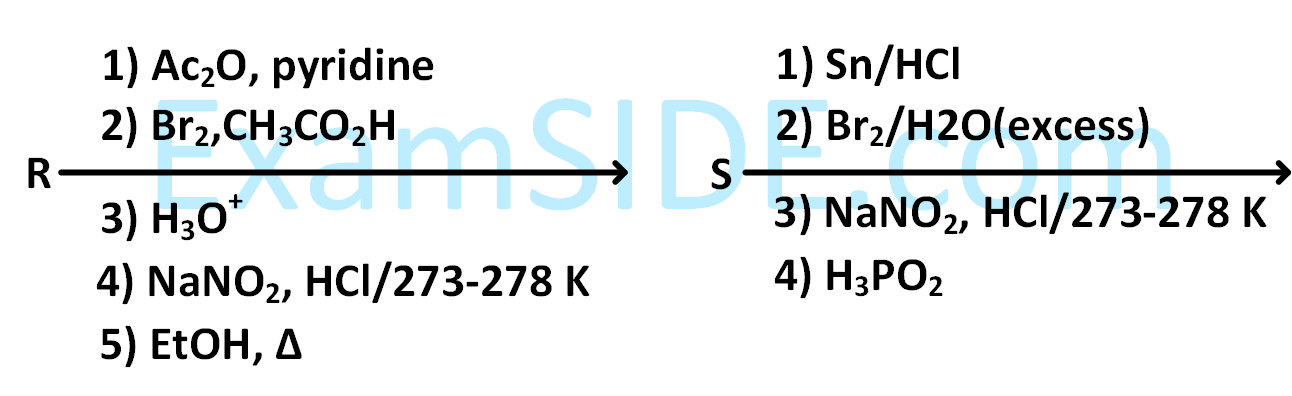
(Use Molar masses (in g mol$$-$$1) of H, C, N, O, Cl and Sn as 1, 12, 14, 16, 35 and 119, respectively).
The value of x is _________.
(Use Molar masses (in g mol$$-$$1) of H, C, N, O, Cl and Sn as 1, 12, 14, 16, 35 and 119, respectively).
The value of y is _________.

