1
JEE Advanced 2025 Paper 2 Online
Numerical
+4
-0

An ideal monatomic gas of $n$ moles is taken through a cycle $W X Y Z W$ consisting of consecutive adiabatic and isobaric quasi-static processes, as shown in the schematic $V-T$ diagram. The volume of the gas at $W, X$ and $Y$ points are, $64 \mathrm{~cm}^3, 125 \mathrm{~cm}^3$ and $250 \mathrm{~cm}^3$, respectively. If the absolute temperature of the gas $T_W$ at the point $W$ is such that $n R T_W=1 \mathrm{~J}$ ( $R$ is the universal gas constant), then the amount of heat absorbed (in J ) by the gas along the path $X Y$ is ___________.
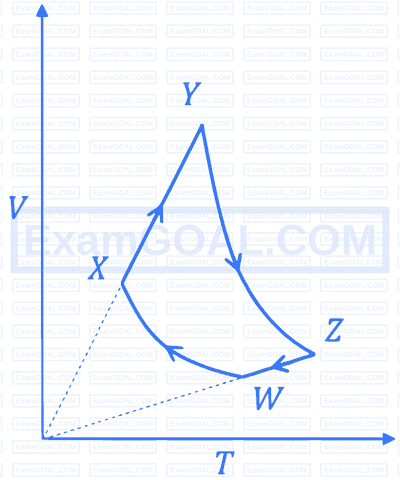

Your input ____
2
JEE Advanced 2025 Paper 2 Online
Numerical
+4
-0

The left and right compartments of a thermally isolated container of length $L$ are separated by a thermally conducting, movable piston of area $A$. The left and right compartments are filled with $\frac{3}{2}$ and 1 moles of an ideal gas, respectively. In the left compartment the piston is attached by a spring with spring constant $k$ and natural length $\frac{2 L}{5}$. In thermodynamic equilibrium, the piston is a distance $\frac{L}{2}$ from the left and right edges of the container as shown in the figure. Under the above conditions, if the pressure in the right compartment is $P=\frac{k L}{A} \alpha$, then the value of $\alpha$ is __________.
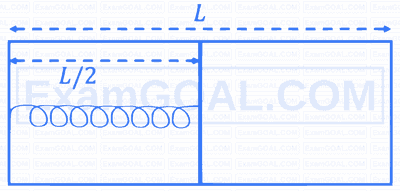

Your input ____
3
JEE Advanced 2025 Paper 1 Online
Numerical
+4
-0

Two identical plates P and Q , radiating as perfect black bodies, are kept in vacuum at constant absolute temperatures $\mathrm{T}_{\mathrm{P}}$ and $\mathrm{T}_{\mathrm{Q}}$, respectively, with $\mathrm{T}_{\mathrm{Q}}<\mathrm{T}_{\mathrm{P}}$, as shown in Fig. 1. The radiated power transferred per unit area from P to Q is $W_0$. Subsequently, two more plates, identical to P and Q , are introduced between P and Q, as shown in Fig. 2. Assume that heat transfer takes place only between adjacent plates. If the power transferred per unit area in the direction from $P$ to $Q$ (Fig. 2) in the steady state is $W_S$, then the ratio $\frac{W_0}{W_S}$ is ________.

Your input ____
4
JEE Advanced 2024 Paper 2 Online
Numerical
+4
-0

A spherical soap bubble inside an air chamber at pressure $P_0=10^5 \mathrm{~Pa}$ has a certain radius so that the excess pressure inside the bubble is $\Delta P=144 \mathrm{~Pa}$. Now, the chamber pressure is reduced to $8 P_0 / 27$ so that the bubble radius and its excess pressure change. In this process, all the temperatures remain unchanged. Assume air to be an ideal gas and the excess pressure $\Delta P$ in both the cases to be much smaller than the chamber pressure. The new excess pressure $\Delta P$ in $\mathrm{Pa}$ is ______.
Your input ____
Questions Asked from Heat and Thermodynamics (Numerical)
Number in Brackets after Paper Indicates No. of Questions
JEE Advanced 2025 Paper 2 Online (2)
JEE Advanced 2025 Paper 1 Online (1)
JEE Advanced 2024 Paper 2 Online (1)
JEE Advanced 2024 Paper 1 Online (1)
JEE Advanced 2023 Paper 2 Online (3)
JEE Advanced 2023 Paper 1 Online (1)
JEE Advanced 2021 Paper 2 Online (2)
JEE Advanced 2021 Paper 1 Online (1)
JEE Advanced 2020 Paper 2 Offline (3)
JEE Advanced 2020 Paper 1 Offline (1)
JEE Advanced 2018 Paper 2 Offline (1)
JEE Advanced 2016 Paper 1 Offline (1)
JEE Advanced 2015 Paper 1 Offline (1)
JEE Advanced 2014 Paper 1 Offline (1)
IIT-JEE 2011 Paper 1 Offline (1)
IIT-JEE 2010 Paper 2 Offline (1)
IIT-JEE 2010 Paper 1 Offline (2)
IIT-JEE 2009 Paper 2 Offline (1)
JEE Advanced Subjects
Physics
Mechanics
Units & Measurements Motion Laws of Motion Work Power & Energy Impulse & Momentum Rotational Motion Properties of Matter Heat and Thermodynamics Simple Harmonic Motion Waves Gravitation
Electricity
Electrostatics Current Electricity Capacitor Magnetism Electromagnetic Induction Alternating Current Electromagnetic Waves
Optics
Modern Physics
Chemistry
Physical Chemistry
Some Basic Concepts of Chemistry Structure of Atom Redox Reactions Gaseous State Chemical Equilibrium Ionic Equilibrium Solutions Thermodynamics Chemical Kinetics and Nuclear Chemistry Electrochemistry Solid State Surface Chemistry
Inorganic Chemistry
Periodic Table & Periodicity Chemical Bonding & Molecular Structure Isolation of Elements Hydrogen s-Block Elements p-Block Elements d and f Block Elements Coordination Compounds Salt Analysis
Organic Chemistry
Mathematics
Algebra
Quadratic Equation and Inequalities Sequences and Series Mathematical Induction and Binomial Theorem Matrices and Determinants Permutations and Combinations Probability Vector Algebra 3D Geometry Statistics Complex Numbers
Trigonometry
Coordinate Geometry
Calculus