Alcohols, Phenols and Ethers · Chemistry · JEE Advanced
MCQ (Single Correct Answer)

The correct statement about $\mathbf{S}$ is :
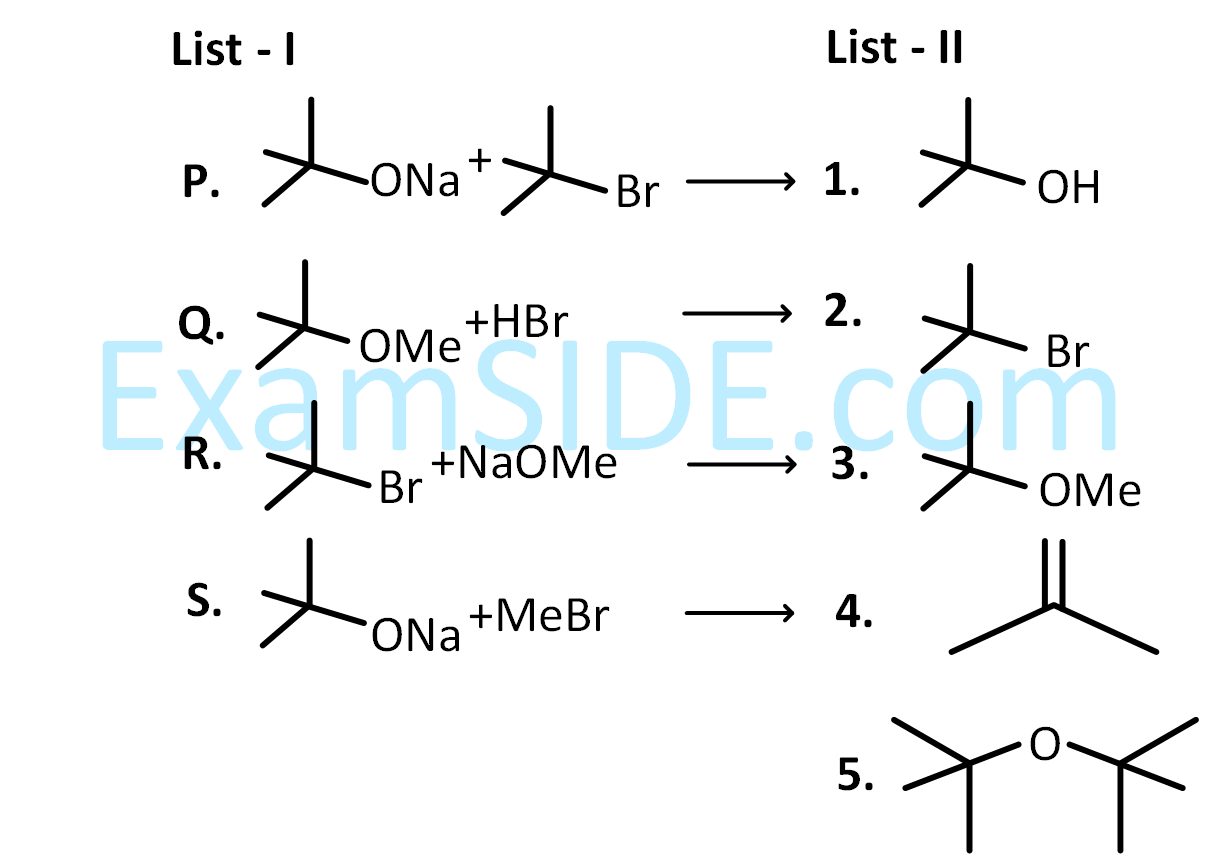
Match the reaction in LIST-I with one or more products in LIST-II and choose the correct option.
For the identification of $$\beta$$-naphthol using dye test, it is necessary to use
The acidic hydrolysis of ether (X) shown below is fastest when

Match the chemical conversions in List I with the appropriate reagents in List II and select the correct answer using the code given below the lists :

The compound that does NOT liberate CO2, on treatment with aqueous sodium bicarbonate solution, is
Match the reactions in Column I with appropriate types of steps/reactive intermediate involved in these reactions as given in Column II :
| Column I | Column II | ||
|---|---|---|---|
| (A) |  |
(P) | Nucleophilic substitution |
| (B) |  |
(Q) | Electrophilic substitution |
| (C) |  |
(R) | Dehydration |
| (D) |  |
(S) | Nucleophilic |
| (T) | Carbanion |
In the reaction

the products are :
Compound H is formed by the reaction of
The structure of compound I is :
The structures of compound J, K and L, respectively, are:
MCQ (More than One Correct Answer)
For the reaction sequence given below, the correct statement(s) is(are)

Reaction of iso-propylbenzene with $\mathrm{O}_2$ followed by the treatment with $\mathrm{H}_3 \mathrm{O}^{+}$forms phenol and a by-product $\mathbf{P}$. Reaction of $\mathbf{P}$ with 3 equivalents of $\mathrm{Cl}_2$ gives compound $\mathbf{Q}$. Treatment of $\mathbf{Q}$ with $\mathrm{Ca}(\mathrm{OH})_2$ produces compound $\mathbf{R}$ and calcium salt $\mathbf{S}$.
The correct statement(s) regarding $\mathbf{P}, \mathbf{Q}, \mathbf{R}$ and $\mathbf{S}$ is(are)



The observed pattern of electrophilic substitution can be explained by
In the following reaction, the product/products formed is/are

The major product(s) of the following reaction is(are)

In the reaction

The intermediate(s) is(are)
Numerical
The reaction sequence given below is carried out with 16 moles of X. The yield of the major product in each step is given below the product in parentheses. The amount (in grams) of S produced is ______.

Use: Atomic mass (in amu): H = 1, C = 12, O = 16, Br = 80
The number of hydroxyl group(s) in Q is ___________.

The number of resonance structure for N is _________.
