Structure of Atom · Chemistry · JEE Advanced
MCQ (Single Correct Answer)
1
According to Bohr's model, the highest kinetic energy is associated with the electron in the
JEE Advanced 2024 Paper 2 Online
2
Consider the Bohr's model of a one-electron atom where the electron moves around the nucleus. In the following List-I contains some quantities for the nth orbit of the atom and List-II contains options showing how they depend on n.
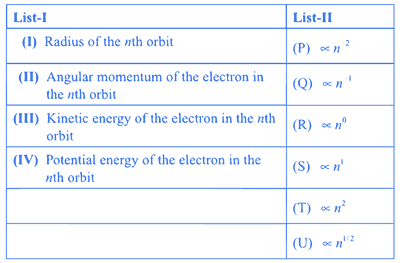
Which of the following options has the correct combination considering List-I and List-II?

Which of the following options has the correct combination considering List-I and List-II?
JEE Advanced 2019 Paper 2 Offline
3
Consider the Bohr's model of a one-electron atom where the electron moves around the nucleus. In the following List-I contains some quantities for the nth orbit of the atom and List-II contains options showing how they depend on n.

Which of the following options has the correct combination considering List-I and List-II?

Which of the following options has the correct combination considering List-I and List-II?
JEE Advanced 2019 Paper 2 Offline
4
For $$H{e^ + }$$ ion, the only INCORRECT combination is
JEE Advanced 2017 Paper 1 Offline
5
For hydrogen atom, the only CORRECT combination is :
JEE Advanced 2017 Paper 1 Offline
6
For the given orbital in Column 1, the only CORRECT combination for any hydrogen-like species is :
JEE Advanced 2017 Paper 1 Offline
7
P is the probability of finding the 1s electron of hydrogen atom in a spherical shell of infinitesimal thickness, dr, at a distance r from the nucleus. The volume of this shell is $$4\pi r^2dr$$. The quantitative ketch of the dependence of P on r is
JEE Advanced 2016 Paper 1 Offline
8
The kinetic energy of an electron in the second Bohr orbit of a hydrogen atom is [$$\alpha_0$$ is Bohr radius]
IIT-JEE 2012 Paper 1 Offline
9
The hydrogen like species Li2+ is in a spherically symmetric state S1 with one radial node. Upon absorbing light the ion undergoes transition to a state S2. The state S2 has one radial node and its energy is equal to the ground state energy of the hydrogen atom.
The state S1 is :
The state S1 is :
IIT-JEE 2010 Paper 2 Offline
10
The hydrogen like species Li2+ is in a spherically symmetric state S1 with one radial node. Upon absorbing light the ion undergoes transition to a state S2. The state S2 has one radial node and its energy is equal to the ground state energy of the hydrogen atom.
Energy of the state S1 in units of the hydrogen atom ground state energy is:
Energy of the state S1 in units of the hydrogen atom ground state energy is:
IIT-JEE 2010 Paper 2 Offline
11
The hydrogen like species Li2+ is in a spherically symmetric state S1 with one radial node. Upon absorbing light the ion undergoes transition to a state S2. The state S2 has one radial node and its energy is equal to the ground state energy of the hydrogen atom.
The orbital angular momentum quantum number of the state S2 is
The orbital angular momentum quantum number of the state S2 is
IIT-JEE 2010 Paper 2 Offline
12
Match the entries in Column I with the correctly related quantum number(s) in Column II. Indicate your answer by darkening the appropriate bubbles of the 4 $$\times$$ 4 matrix given in the ORS.
| Column I | Column II | ||
|---|---|---|---|
| (A) | Orbital angular momentum of the electron in a hydrogen-like atomic orbital. | (P) | Principal quantum number |
| (B) | A hydrogen-like one-electron wave function obeying Pauli's principle. | (Q) | Azimuthal quantum number |
| (C) | Shape, size and orientation of hydrogen like atomic orbitals. | (R) | Magnetic quantum number |
| (D) | Probability density of electron at the nucleus in hydrogen-like atom. | (S) | Electron spin quantum number |
IIT-JEE 2008 Paper 2 Offline
13
STATEMENT - 1 : The plot of atomic number (y-axis) versus number of neutrons (x-axis) for stable nuclei shows a curvature towards x-axis from the line of 45o slope as the atomic number is increased.
STATEMENT - 2 : Proton-proton electrostatic repulsions begin to overcome attractive forces involving protons and neutrons in heavier nuclides
STATEMENT - 2 : Proton-proton electrostatic repulsions begin to overcome attractive forces involving protons and neutrons in heavier nuclides
IIT-JEE 2008 Paper 1 Offline
14
According to Bohr's theory
En = Total energy, Kn = Kinetic Energy, Vn = Potential Energy, rn = Radius of nth orbit
Match the following
Column I
(A) Vn/Kn = ?
(B) If radius of nth orbit $$ \propto E_n^x,x = ?$$
(C) Angular momentum in lowest orbital
(D) $${1 \over {{r_n}}} \propto {Z^y},y = ?$$
Column II
(p) 0
(q) -1
(r) -2
(s) 1
En = Total energy, Kn = Kinetic Energy, Vn = Potential Energy, rn = Radius of nth orbit
Match the following
Column I
(A) Vn/Kn = ?
(B) If radius of nth orbit $$ \propto E_n^x,x = ?$$
(C) Angular momentum in lowest orbital
(D) $${1 \over {{r_n}}} \propto {Z^y},y = ?$$
Column II
(p) 0
(q) -1
(r) -2
(s) 1
IIT-JEE 2006
15
The number of radial nodes of 3s and 2p orbitals are respectively
IIT-JEE 2005 Screening
16
The radius of which of the following orbit is same as that of the first Bohr's orbit of hydrogen atom?
IIT-JEE 2004 Screening
17
Which of the following pairs are isoelectronic and isostructural?
$$NO_3^-$$, $$CO_3^{2-}$$, $$ClO_3^-$$, SO3
$$NO_3^-$$, $$CO_3^{2-}$$, $$ClO_3^-$$, SO3
IIT-JEE 2003 Screening
18
If the nitrogen atom has electronic configuration 1s7, it would have energy lower than that of the normal ground state configuration 1s22s22p3, because the electrons would be closer to the nucleus. Yet 1s7 is not observed because it violates
IIT-JEE 2002 Screening
19
Rutherford's experiment, which established the nuclear model of the atom, used a beam of
IIT-JEE 2002 Screening
20
The quantum numbers +1/2 and -1/2 for the electron spin represent
IIT-JEE 2001 Screening
21
The wavelength associated with a golf ball weighing 200 g and moving at speed of 5 m/h is of the order
IIT-JEE 2001 Screening
22
The number of nodal planes in a px orbital is
IIT-JEE 2000 Screening
23
The electronic configuration of an element is 1s2, 2s2 2p6, 3s2 3p6 3d5, 4s1 This represents its
IIT-JEE 2000 Screening
24
The electrons, identified by quantum numbers n and l, (i) n = 4, l = 1, (ii) n = 4, l = 0, (iii) n = 3, l = 2 and (iv) n = 3, l = 1 can be placed in order of increasing energy, from the lowest to highest, as
IIT-JEE 1999
25
ASSERTION:
Nuclide $${}_{13}^{30}Al$$ is less stable than $${}_{20}^{40}Ca$$
REASON:
Nuclides having odd number of protons and neutrons are generally unstable.
Nuclide $${}_{13}^{30}Al$$ is less stable than $${}_{20}^{40}Ca$$
REASON:
Nuclides having odd number of protons and neutrons are generally unstable.
IIT-JEE 1998
26
The orbital diagram in which the Aufbau principle is violated is
IIT-JEE 1998
27
For a d-electron the orbital angular momentum is
IIT-JEE 1997
28
The orbital angular momentum of an electron in 2s orbital is
IIT-JEE 1996
29
A 3p orbital has
IIT-JEE 1995 Screening
30
Which of the following does not characterise X-rays?
IIT-JEE 1992
31
Which of the following relates to photons both as wave motion and as a stream of particles?
IIT-JEE 1992
32
The correct set of quantum numbers for the unpaired electron of chlorine atom is
IIT-JEE 1989
33
The correct ground state electronic configuration of chromium atom is
IIT-JEE 1989
34
The outermost electronic configuration of the most electronegative element is
IIT-JEE 1988
35
The wavelength of a spectral line for an electronic transition is inversely related to :
IIT-JEE 1988
36
The triad of nuclei that is isotonic is
IIT-JEE 1988
37
The sum of the number of neutrons and proton in the isotope of hydrogen is
IIT-JEE 1986
38
Rutherford's alpha particle scattering experiment eventually led to the conclusion that:
IIT-JEE 1986
39
Which one of the following sets of quantum numbers represents an impossible arrangement?
IIT-JEE 1986
40
The ratio of the energy of a photon of 2000Å wavelength to that 4000Å radiation is
IIT-JEE 1986
41
Bohr Model can explain
IIT-JEE 1985
42
The radius of an atomic nucleus is of the order of
IIT-JEE 1985
43
Electromangnetic radiation with maximum wavelength is:
IIT-JEE 1985
44
Which electronic level would allow the hydrogen atom to absorb a photon but not to emit a photon?
IIT-JEE 1984
45
The increasing order (lowest first) for the values of e/m (charges/mass) for electron (e), proton (p), neutron (n) and alpha particle ($$\alpha$$) is:
IIT-JEE 1984
46
Correct set of four quantum numbers for the valence (outermost) electron of rubidium (Z = 37) is
IIT-JEE 1984
47
Rutherford's scattering experiment is related to the size of the
IIT-JEE 1983
48
Any p-orbital can accommodate upto
IIT-JEE 1983
49
The principal quantum number of an atom is related to the
IIT-JEE 1983
50
The ion that is isoelectronic with CO is
IIT-JEE 1982
51
Rutherford's experiment on scattering of $$\alpha-particles$$ showed for the first time that the atom has
IIT-JEE 1981
52
The number of neutrons in dipositive zinc ion with mass number 70 is
IIT-JEE 1979
MCQ (More than One Correct Answer)
1
Among the following, the correct statement(s) for electrons in an atom is(are)
JEE Advanced 2024 Paper 1 Online
2
For diatomic molecules, the correct statement(s) about the molecular orbitals formed by the overlap of two $2 p_{z}$ orbitals is(are)
JEE Advanced 2022 Paper 1 Online
3
The ground state energy of hydrogen atom is $$-$$13.6 eV. Consider an electronic state $$\psi $$ of He+ whose energy, azimuthal quantum number and magnetic quantum number are $$-$$3.4 eV, 2 and 0, respectively.
Which of the following statement(s) is(are) true for the state $$\psi $$?
Which of the following statement(s) is(are) true for the state $$\psi $$?
JEE Advanced 2019 Paper 2 Offline
4
Ground state electronic configuration of nitrogen atom can be represented by
IIT-JEE 1999
5
Which of the following statement(s) is (are) correct?
IIT-JEE 1998
6
Decrease in atomic number is observed during
IIT-JEE 1998
7
The energy of an electron in the first Bohr orbit of H atom is -13.6 eV. The possible energy value(s) of the excited state(s) for electrons in Bohr orbits of hydrogen is (are)
IIT-JEE 1998
8
When alpha particles are sent through a thin metal foil, most of them go straight through the foil because
IIT-JEE 1984
9
An isotone of $${}_{32}^{76}Ge$$ is
IIT-JEE 1984
10
Many elements have non-integral atomic masses because:
IIT-JEE 1984
Numerical
1
For $\mathrm{He}^{+}$, a transition takes place from the orbit of radius $105.8 \mathrm{pm}$ to the orbit of radius $26.45 \mathrm{pm}$. The wavelength (in nm) of the emitted photon during the transition is _______.
[Use :
Bohr radius, $\mathrm{a}=52.9 \mathrm{pm}$
Rydberg constant, $R_{\mathrm{H}}=2.2 \times 10^{-18} \mathrm{~J}$
Planck's constant, $\mathrm{h}=6.6 \times 10^{-34} \mathrm{~J} \mathrm{~s}$
Speed of light, $\mathrm{c}=3 \times 10^8 \mathrm{~m} \mathrm{~s}^{-1}$ ]
[Use :
Bohr radius, $\mathrm{a}=52.9 \mathrm{pm}$
Rydberg constant, $R_{\mathrm{H}}=2.2 \times 10^{-18} \mathrm{~J}$
Planck's constant, $\mathrm{h}=6.6 \times 10^{-34} \mathrm{~J} \mathrm{~s}$
Speed of light, $\mathrm{c}=3 \times 10^8 \mathrm{~m} \mathrm{~s}^{-1}$ ]
JEE Advanced 2023 Paper 2 Online
2
Consider a helium (He) atom that absorbs a photon of wavelength 330 nm. The change in the velocity (in cm s$$-$$1) of He atom after the photon absorption is __________.
(Assume : Momentum is conserved when photon is absorbed.
Use : Planck constant = 6.6 $$\times$$ 10$$-$$34 J s, Avogadro number = 6 $$\times$$ 1023 mol$$-$$1, Molar mass of He = 4 g mol$$-$$1)
(Assume : Momentum is conserved when photon is absorbed.
Use : Planck constant = 6.6 $$\times$$ 10$$-$$34 J s, Avogadro number = 6 $$\times$$ 1023 mol$$-$$1, Molar mass of He = 4 g mol$$-$$1)
JEE Advanced 2021 Paper 2 Online
3
The figure below is the plot of potential energy versus internuclear distance (d) of H2 molecule in the electronic ground state. What is the value of the net potential energy E0 (as indicated in the figure) in kJ mol-1, for d = d0 at which the electron-electron repulsion and the nucleus-nucleus repulsion energies are absent? As reference, the potential energy of H atom is taken as zero when its electron and the nucleus are infinitely far apart.
Use Avogadro constant as 6.023 $$ \times $$ 1023 mol-1.

Use Avogadro constant as 6.023 $$ \times $$ 1023 mol-1.

JEE Advanced 2020 Paper 2 Offline
4
Not considering the electronic spin, the degeneracy of the second excited state( n = 3) of H atom is 9, while
the degeneracy of the second excited state of H– is ___________.
JEE Advanced 2015 Paper 1 Offline
5
In an atom, the total number of electrons having quantum numbers n = 4, |ml| = 1 and ms = –1/2 is
JEE Advanced 2014 Paper 1 Offline
6
The atomic masses of He and Ne are 4 and 20 a.m.u., respectively. The value of the de Broglie wavelength
of He gas at –73oC is “M” times that of the de Broglie wavelength of Ne at 727oC. M is
JEE Advanced 2013 Paper 1 Offline
7
The maximum number of electrons that can have principal quantum number, n = 3, and spin quantum number, ms = − 1/2 , is
IIT-JEE 2011 Paper 1 Offline
8
The work function
( φ )
of some metals is listed below. The number of metals which will show
photoelectric effect when light of 300 nm wavelength falls on the metal is
| Metal | Li | Na | K | Mg | Cu | Ag | Fe | Pt | W |
|---|---|---|---|---|---|---|---|---|---|
| Ф (eV) | 2.4 | 2.3 | 2.2 | 3.7 | 4.8 | 4.3 | 4.7 | 6.3 | 4.75 |
IIT-JEE 2011 Paper 1 Offline
9
Find out the number of waves made by a Bohr electron in once complete revolution in its 3rd orbit?
IIT-JEE 1994
10
What is the maximum number of electrons that may be present in all atomic orbitals with principal quantum number 3 and azimuthal quantum number 2?
IIT-JEE 1985
Subjective
1
Find the velocity (ms-1) of electron in first Bohr's orbit of radius a0. Also find the de Broglie's wavelength (in m). Find the orbital angular momentum of 2p orbital of hydrogen atom in units of $$h/2 \pi$$.
IIT-JEE 2005
2
A ball of mass 100 g is moving with 100 ms-1. Find it's wavelength.
IIT-JEE 2004
3
(a) The Schrodinger wave equation for hydrogen atom is
$$$\psi = {1 \over {4\sqrt {2\pi } }}{\left( {{1 \over {{a_0}}}} \right)^{3/2}}\left( {2 - {{{r_0}} \over {{a_0}}}} \right){e^{ - {r_0}/{a_0}}}$$$
Where a0 is Bohr's radius. If the radial node in 2s be at r0, then find r0 in terms of a0.
(b) A baseball having mass 100 g moves with velocity 100 m/s. Determine the value of wavelength of baseball.
$$$\psi = {1 \over {4\sqrt {2\pi } }}{\left( {{1 \over {{a_0}}}} \right)^{3/2}}\left( {2 - {{{r_0}} \over {{a_0}}}} \right){e^{ - {r_0}/{a_0}}}$$$
Where a0 is Bohr's radius. If the radial node in 2s be at r0, then find r0 in terms of a0.
(b) A baseball having mass 100 g moves with velocity 100 m/s. Determine the value of wavelength of baseball.
IIT-JEE 2004
4
Wavelength of high energy transition of H-atoms is 91.2 nm. Calculate the corresponding wavelength of He atoms.
IIT-JEE 2003
5
Calculate the energy required to excite one litre of hydrogen gas at 1 atm and 298 K to the first excited state of atomic hydrogen. The energy for the dissociation of H-H bond 436 kJ mol-1.
IIT-JEE 2000
6
Calculate the wave number for the shortest wavelength transition in the Balmer series of atomic hydrogen.
IIT-JEE 1996
7
Consider the hydrogen atom to be a proton embedded in a cavity of radius a0 (Bohr radius) whose charge is neutralised by the addition of an electron to the cavity in vacuum, infinitely slowly. Estimate the average total energy of an electron in it's ground state in a hydrogen atom as the work done in the above neutralisation process. Also, if the magnitude of the average kinetic energy is half the magnitude of the average potential energy, find the average potential energy.
IIT-JEE 1996
8
Iodine molecule dissociates into atoms after absorbing light of 4500 Å. If one quantum of radiation is absorbed by each molecule, calculate the kinetic energy of iodine atoms. (Bond energy of I2 = 240 kJ mol-1)
IIT-JEE 1995
9
What transition in the hydrogen spectrum would have the same wavelength as the Balmer transition n = 4 to n = 2 of He+ spectrum?
IIT-JEE 1993
10
Estimate the difference in energy between 1st and 2nd Bohr orbit for a hydrogen atom. At what minimum atomic number, a transition from n = 2 to n = 1 energy level would result in the emission of X-rays with $$\lambda = 3.0 \times {10^{ - 8}}$$? Which hydrogen atom like species does this atomic number correspond to?
IIT-JEE 1993
11
According to Bohr's theory, the electronic energy of hydrogen atom in the nth Bohr's orbit is given by $${E_n} = {{ - 21.6 \times {{10}^{ - 19}}} \over {{n^2}}}J$$. Calculate the longest wavelength of light (in Å) that will be needed to remove an electron from the third Bohr orbit of the He+ ion.
IIT-JEE 1990
12
Give reasons why the ground state outermost electronic configuration of silicon is:


IIT-JEE 1985
13
The electron energy in hydrogen atom is given by E = (-21.7 $$\times$$ 10-12)/n2 ergs. Calculate the energy required to remove an electron completely from the n = 2 orbit. What is the longest wavelength (in cm) of light that can be used to cause this transition?
IIT-JEE 1984
14
Calculate the wavelength in Angstrom of the photon that is emitted when an electron in the Bohr orbit, n = 2 returns to the orbit, n = 1 in the hydrogen atom. The ionization potential of the ground state hydrogen atom is 2.17 $$\times$$ 10-11 erg per atom.
IIT-JEE 1982
15
The energy of the electron in the second and the third Bohr's orbits of the hydrogen atom is -5.42 $$\times$$ 10-12 erg and -2.41 $$\times$$ 10-12 erg respectively. Calculate the wavelength (in Å) of the emitted radiation when the electron drops from the third to second orbit.
IIT-JEE 1981
Fill in the Blanks
1
The outermost electronic configuration of Cr is _______.
IIT-JEE 1994
2
The 2px, 2py and 2pz orbitals of atom have identical shapes but differ in their _____.
IIT-JEE 1993
3
Wave functions of electrons in atoms and molecules are called ______.
IIT-JEE 1993
4
The light radiations with discrete quantities of energy are called ______.
IIT-JEE 1993
5
The uncertainty principle and the concept of wave nature of matter were proposed by ______ and ______ respectively. (Heisenberg Schrodinger, Maxwell, de Broglie)
IIT-JEE 1988
6
Elements of the same mass number but of different atomic numbers are known as ____.
IIT-JEE 1983
7
Isotopes of an element differ in the number of ______ in their nuclei.
IIT-JEE 1982
8
When there are two electrons in the same orbital, they have _____ spins.
IIT-JEE 1982
9
The mass of a hydrogen atom is ______ kg.
IIT-JEE 1982
True or False
1
In a given electric field, $$\beta-particles $$ are deflected more than $$\alpha-particles$$ in spite of $$\alpha-particles$$ having larger charge.
IIT-JEE 1993
2
The electron density in the XY plane in 3dx2 - y2 orbital is zero
IIT-JEE 1986
3
Gamma rays are electromagnetic radiations of wavelengths of 10-6 to 10-5 cm
IIT-JEE 1983
4
The energy of the electron in the 3d-orbital is less than that in the 4s-orbital in the hydrogen atom.
IIT-JEE 1983
5
The outer electronic configuration of the ground state chromium atom is 3d4 4s2
IIT-JEE 1982