
The temperature of water fed into the device cannot exceed 30°C and the entire stored 120 litres of water is initially cooled to 10°C. The entire system is thermally insulated. The minimum value of P (in watts) for which the device can be operated for 3 hours is :
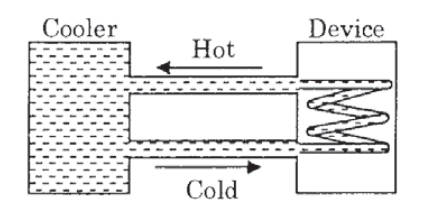
(Specific heat of water is 4.2 kJ kg−1 K−1 and the density of water is 1000 kg m−3)
In the figure a container is shown to have a movable (without friction) piston on top. The container and the piston are all made of perfectly insulating material allowing no heat transfer between outside and inside the container. The container is divided into two compartments by a rigid partition made of a thermally conducting material that allows slow transfer of heat. The lower compartment of the container is filled with 2 moles of an ideal monatomic gas at 700 K and the upper compartment is filled with 2 moles of an ideal diatomic gas at 400 K. The heat capacities per mole of an ideal monatomic gas are $${C_v} = {3 \over 2}R$$, $${C_p} = {5 \over 2}R$$, and those for an ideal diatomic gas are $${C_v} = {5 \over 2}R$$, $${C_p} = {7 \over 2}R$$.

Consider the partition to be rigidly fixed so that it does not move. When equilibrium is achieved, the final temperature of the gases will be
In the figure a container is shown to have a movable (without friction) piston on top. The container and the piston are all made of perfectly insulating material allowing no heat transfer between outside and inside the container. The container is divided into two compartments by a rigid partition made of a thermally conducting material that allows slow transfer of heat. The lower compartment of the container is filled with 2 moles of an ideal monatomic gas at 700 K and the upper compartment is filled with 2 moles of an ideal diatomic gas at 400 K. The heat capacities per mole of an ideal monatomic gas are $${C_v} = {3 \over 2}R$$, $${C_p} = {5 \over 2}R$$, and those for an ideal diatomic gas are $${C_v} = {5 \over 2}R$$, $${C_p} = {7 \over 2}R$$.

Now consider the partition to be free to move without friction so that the pressure of gases in both compartments is the same. Then total work done by the gases till the time they achieve equilibrium will be