Aldehydes, Ketones and Carboxylic Acids · Chemistry · JEE Advanced
MCQ (Single Correct Answer)
Monocyclic compounds $\mathbf{P}, \mathbf{Q}, \mathbf{R}$ and $\mathbf{S}$ are the major products formed in the reaction sequences given below.

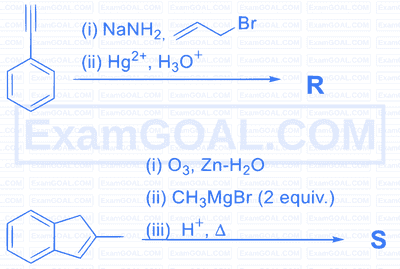
The product having the highest number of unsaturated carbon atom(s) is :
In the following reaction sequence, the major product $\mathbf{Q}$ is

List-I contains various reaction sequences and List-II contains the possible products. Match each entry in List-I with the appropriate entry in List-II and choose the correct option.
| List-I | List-II |
|---|---|

|

|

|

|

|

|

|

|

|
| List - I | List - II |
|---|---|
| (P) Etard reaction | (1) Acetophenone $\stackrel{\mathrm{Zn}-\mathrm{Hg}, \mathrm{HCl}}{\longrightarrow}$ |
| (Q) Gattermann reaction | (2) $$ \text { Toluene } \underset{\text { (ii) } \mathrm{SOCl}_2}{\stackrel{\text { (i) } \mathrm{KMnO}_4, \mathrm{KOH}, \Delta}{\longrightarrow}} $$ |
| (R) Gattermann-Koch reaction | (3) $$ \text { Benzene } \underset{\text { anhyd. } \mathrm{AlCl}_3}{\stackrel{\mathrm{CH}_3 \mathrm{Cl}}{\longrightarrow}} $$ |
| (S) Rosenmund reduction | (4) $$ \text { Aniline } \underset{273-278 \mathrm{~K}}{\stackrel{\mathrm{NaNO}_2 / \mathrm{HCl}}{\longrightarrow}} $$ |
| (5) $$ \text { Phenol } \stackrel{\mathrm{Zn}, \Delta}{\longrightarrow} $$ |

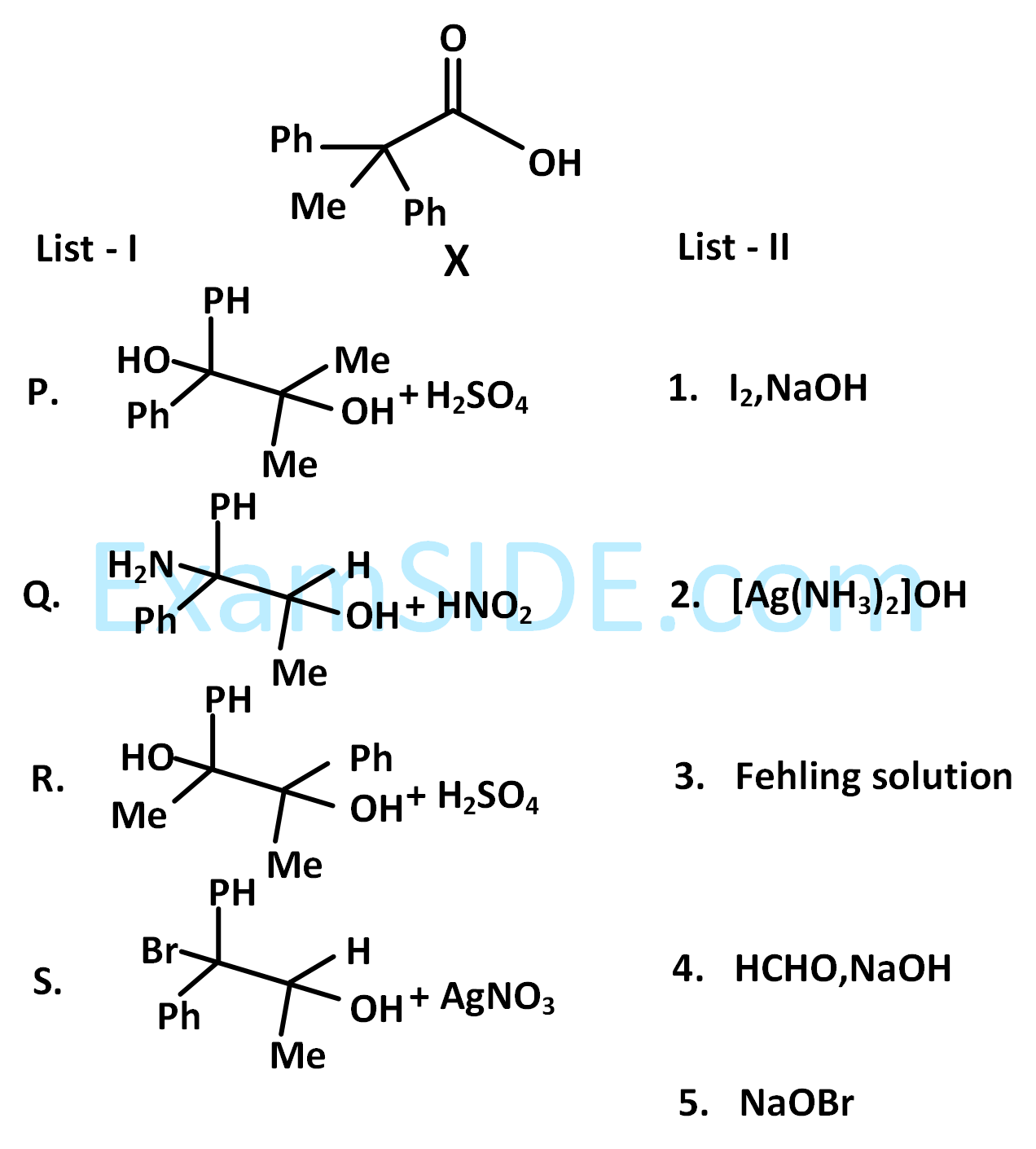
The correct option is

The compound S is

The compound R is
The correct order of acidity for the following compounds is

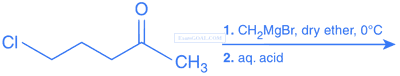
The major product of the following reaction sequence is:

The major product in the following reaction is
Different possible thermal decomposition pathways for peroxyesters are shown below. Match each pathway from List I with an appropriate structure from List II and select the correct answer using the code given below the lists.


Compounds formed from P and Q are, respectively,
In the following reaction sequences V and W are, respectively,

The major product H of the given reaction sequence is
$$C{H_3} - C{H_2} - CO - C{H_3}\buildrel {\overline C N} \over \longrightarrow G\mathrel{\mathop{\kern0pt\longrightarrow} \limits_{Heat}^{95\% \,{H_2}S{O_4}}} H$$
The compound that undergoes decarboxylation most readily under mild condition is
The compound K is
The carboxyl functional group ($$-$$COOH) is present in
The number of aldol reactions that occur in the given transformation are

The number of optically active products obtained from the complete ozonolysis of the given compound is ______.

The structure of compound P is
The structure of the compound Q is
Match each of the compounds given in Column I with the reaction(s), that they can undergo, given in Column II.
| Column I | Column II | ||
|---|---|---|---|
| (A) |  |
(P) | Nucleophilic substitution |
| (B) |  |
(Q) | Elimination |
| (C) |  |
(R) | Nucleophilic addition |
| (D) |  |
(S) | Esterification with acetic anhydride |
| (T) | Dehydrogenation |
The structure of the carbonyl compound P is
The structures of the products Q and R, respectively, are
The structure of the product S is
In the following reaction sequence, the correct structure of E, F and G are :

The structure of the product I is :
The structure of compounds J and K, respectively, are:
The structure of product L is :
Numerical
An organic compound $\mathbf{P}$ having molecular formula $\mathrm{C}_6 \mathrm{H}_6 \mathrm{O}_3$ gives ferric chloride test and does not have intramolecular hydrogen bond. The compound $\mathbf{P}$ reacts with 3 equivalents of $\mathrm{NH}_2 \mathrm{OH}$ to produce oxime $\mathbf{Q}$. Treatment of $\mathbf{P}$ with excess methyl iodide in the presence of $\mathrm{KOH}$ produces compound $\mathbf{R}$ as the major product. Reaction of $\mathbf{R}$ with excess iso-butylmagnesium bromide followed by treatment with $\mathrm{H}_3 \mathrm{O}^{+}$gives compound $\mathbf{S}$ as the major product.
The total number of methyl $\left(-\mathrm{CH}_3\right)$ group(s) in compound $\mathbf{S}$ is ___.
Complete reaction of acetaldehyde with excess formaldehyde, upon heating with conc. $\mathrm{NaOH}$ solution, gives $\mathbf{P}$ and $\mathbf{Q}$. Compound $\mathbf{P}$ does not give Tollens' test, whereas $\mathbf{Q}$ on acidification gives positive Tollens' test. Treatment of $\mathbf{P}$ with excess cyclohexanone in the presence of catalytic amount of $p$-toluenesulfonic acid (PTSA) gives product $\mathbf{R}$.
Sum of the number of methylene groups $\left(-\mathrm{CH}_2-\right)$ and oxygen atoms in $\mathbf{R}$ is __________.


What is the degree of unsaturation of Q?
(Atomic weights in $$g\,mo{l^{ - 1}}:H = 1,C = 12,$$ $$N = 14,O = 16,$$ $$Br = 80.$$. The yield (%) corresponding to the product in each step is given in the parenthesis)
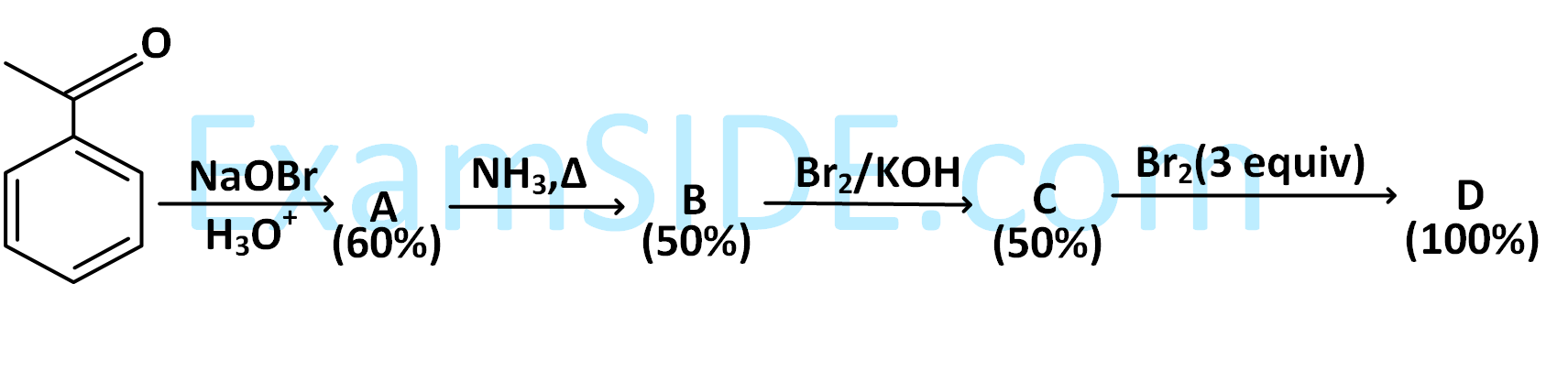
Among the following the number of reactions that produces benzaldehyde is _________.

The total number of carboxylic acid groups is the product P is _________.

In the scheme given below, the total number of intra molecular aldol condensation products formed from Y is ____________.

Amongst the following, the total number of compounds soluble in aqueous NaOH is _________.

MCQ (More than One Correct Answer)


The correct statement(s) about $\mathbf{P}, \mathbf{Q}, \mathbf{R}$, and $\mathbf{S}$ is(are) :


The correct statement(s) about $\mathbf{P}, \mathbf{Q}, \mathbf{R}$, and $\mathbf{S}$ is(are) :
Considering the reaction sequence given below, the correct statement(s) is(are)


The correct structure of
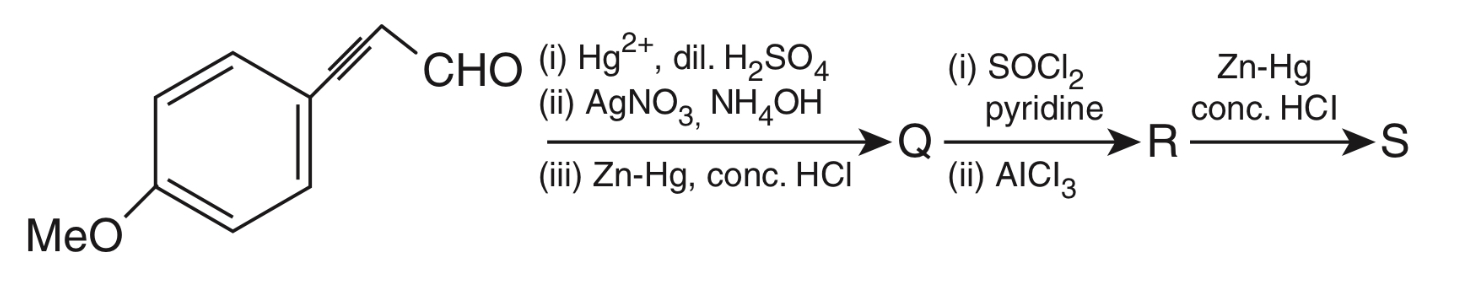
Consider Q, R and S as major products.

The option(s) with suitable combination of $$P$$ and $$R,$$ respectively, is (are)
Reagent(s) which can be used to bring about the following transformation is(are)

The correct statements about of the following reaction sequence is (are)
Cumene (C9H12) $$\mathrel{\mathop{\kern0pt\longrightarrow}
\limits_{(ii)\,{H_3}{O^ + }}^{(i)\,{O_2}}} $$ P $$\mathrel{\mathop{\kern0pt\longrightarrow}
\limits_{}^{CHC{l_3}/NaOH}} $$ Q (major) + R (minor)
Q $$\mathrel{\mathop{\kern0pt\longrightarrow}
\limits_{PhC{H_2}Br}^{NaOH}} $$ S
In the following reactions

Compound X is
In the following reactions

The major compound Y is
The major product of the following reaction is

After completion of the reactions (I and II), the organic compound(s) in the reaction mixtures is(are)

With reference to the scheme given, which of the given statement(s) about T, U, V and W is(are) correct?

Identify the binary mixtures that can be separated into individual compounds, by differential extraction, as shown in the given scheme.
