1
JEE Advanced 2018 Paper 2 Offline
MCQ (Single Correct Answer)
+3
-1

One mole of a monatomic ideal gas undergoes four thermodynamic processes as shown schematically in the $$PV$$-diagram below. Among these four processes, one is isobaric, one is isochoric, one is isothermal and one is adiabatic. Match the processes mentioned in List-I with the corresponding statements in List-II.
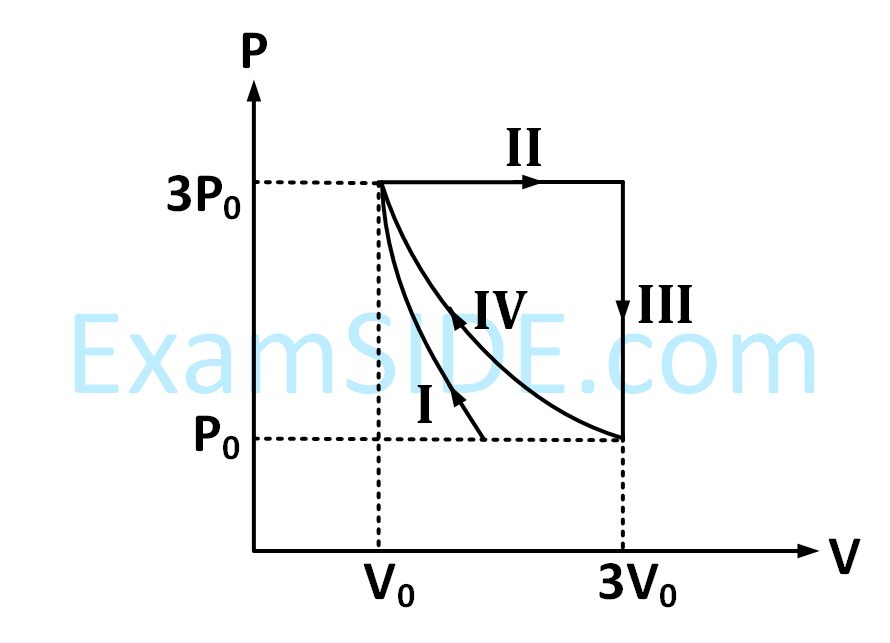

| LIST - I | LIST - II | ||
|---|---|---|---|
| P. | In process I | 1. | Work done by the gas is zero |
| Q. | In process II | 2. | Temperature of the gas remains unchanged |
| R. | In process III | 3. | No heat is exchanged between the gas and its surroundings |
| S. | In process IV | 4. | Work done by the gas is 6P0V0 |
2
JEE Advanced 2017 Paper 1 Offline
MCQ (Single Correct Answer)
+3
-0.75

An ideal gas is undergoing a cyclic thermodynamic process in different ways as shown in the corresponding $$P-V$$ diagram in column 3 of the table. Consider only the path from state $$1$$ to state $$2.$$ $$W$$ denotes the corresponding work done on the system. The equations and plots in the table have standard notations as used in thermodynamic processes. Here $$Y$$ is the ratio of heat capacities at constant pressure and constant volume. The number of moles in the gas is $$n.$$




Which one of the following options correctly represents a thermodynamic process that is used as a correction in the determination of the speed of sound in an ideal gas?
3
JEE Advanced 2017 Paper 1 Offline
MCQ (Single Correct Answer)
+3
-0.75

An ideal gas is undergoing a cyclic thermodynamic process in different ways as shown in the corresponding $$P-V$$ diagram in column 3 of the table. Consider only the path from state $$1$$ to state $$2.$$ $$W$$ denotes the corresponding work done on the system. The equations and plots in the table have standard notations as used in thermodynamic processes. Here $$Y$$ is the ratio of heat capacities at constant pressure and constant volume. The number of moles in the gas is $$n.$$




Which one of the following options is the correct combination?
4
JEE Advanced 2017 Paper 1 Offline
MCQ (Single Correct Answer)
+3
-0.75

An ideal gas is undergoing a cyclic thermodynamic process in different ways as shown in the corresponding $$P-V$$ diagram in column 3 of the table. Consider only the path from state $$1$$ to state $$2.$$ $$W$$ denotes the corresponding work done on the system. The equations and plots in the table have standard notations as used in thermodynamic processes. Here $$Y$$ is the ratio of heat capacities at constant pressure and constant volume. The number of moles in the gas is $$n.$$




Which of the following options is the only correct representation of a process in which
$$\Delta U = \Delta Q - P\Delta V$$?
$$\Delta U = \Delta Q - P\Delta V$$?
Questions Asked from Heat and Thermodynamics (MCQ (Single Correct Answer))
Number in Brackets after Paper Indicates No. of Questions
JEE Advanced 2024 Paper 1 Online (1)
JEE Advanced 2023 Paper 2 Online (1)
JEE Advanced 2023 Paper 1 Online (2)
JEE Advanced 2022 Paper 1 Online (1)
JEE Advanced 2021 Paper 2 Online (2)
JEE Advanced 2021 Paper 1 Online (1)
JEE Advanced 2019 Paper 2 Offline (2)
JEE Advanced 2018 Paper 2 Offline (1)
JEE Advanced 2017 Paper 1 Offline (3)
JEE Advanced 2016 Paper 2 Offline (2)
JEE Advanced 2016 Paper 1 Offline (1)
JEE Advanced 2014 Paper 2 Offline (3)
JEE Advanced 2013 Paper 2 Offline (1)
JEE Advanced 2013 Paper 1 Offline (2)
IIT-JEE 2012 Paper 2 Offline (1)
IIT-JEE 2012 Paper 1 Offline (2)
IIT-JEE 2011 Paper 1 Offline (1)
IIT-JEE 2011 Paper 2 Offline (1)
IIT-JEE 2010 Paper 1 Offline (1)
IIT-JEE 2009 Paper 2 Offline (1)
IIT-JEE 2008 Paper 2 Offline (1)
IIT-JEE 2008 Paper 1 Offline (1)
IIT-JEE 2007 Paper 2 Offline (1)
JEE Advanced Subjects
Physics
Mechanics
Units & Measurements Motion Laws of Motion Work Power & Energy Impulse & Momentum Rotational Motion Properties of Matter Heat and Thermodynamics Simple Harmonic Motion Waves Gravitation
Electricity
Electrostatics Current Electricity Capacitor Magnetism Electromagnetic Induction Alternating Current Electromagnetic Waves
Optics
Modern Physics
Chemistry
Physical Chemistry
Some Basic Concepts of Chemistry Structure of Atom Redox Reactions Gaseous State Chemical Equilibrium Ionic Equilibrium Solutions Thermodynamics Chemical Kinetics and Nuclear Chemistry Electrochemistry Solid State Surface Chemistry
Inorganic Chemistry
Periodic Table & Periodicity Chemical Bonding & Molecular Structure Isolation of Elements Hydrogen s-Block Elements p-Block Elements d and f Block Elements Coordination Compounds Salt Analysis
Organic Chemistry
Mathematics
Algebra
Quadratic Equation and Inequalities Sequences and Series Mathematical Induction and Binomial Theorem Matrices and Determinants Permutations and Combinations Probability Vector Algebra 3D Geometry Statistics Complex Numbers
Trigonometry
Coordinate Geometry
Calculus