Biomolecules · Chemistry · JEE Advanced
Numerical
A linear octasaccharide (molar mass $=1024 \mathrm{~g} \mathrm{~mol}^{-1}$ ) on complete hydrolysis produces three monosaccharides: ribose, 2-deoxyribose and glucose. The amount of 2-deoxyribose formed is $58.26 \%(\mathrm{w} / \mathrm{w})$ of the total amount of the monosaccharides produced in the hydrolyzed products. The number of ribose unit(s) present in one molecule of octasaccharide is $\qquad$ .
Use: Molar mass $\left(\right.$ in g $\left.\mathrm{mol}^{-1}\right)$ : ribose $=150,2$-deoxyribose $=134$, glucose $=180$;
Atomic mass (in amu): $\mathrm{H}=1, \mathrm{O}=16$
For a double strand DNA, one strand is given below:

The amount of energy required to split the double strand DNA into two single strands is _______ kcal $\operatorname{mol}^{-1}$.
[Given: Average energy per H-bond for A-T base pair $=1.0 ~\mathrm{kcal}~ \mathrm{mol}^{-1}$, G-C base pair $=1.5 ~\mathrm{kcal}$ $\mathrm{mol}^{-1}$, and A-U base pair $=1.25 ~\mathrm{kcal} ~\mathrm{mol}^{-1}$. Ignore electrostatic repulsion between the phosphate groups.]

If the absolute values of the net charge of the peptide at $\mathrm{pH}$ $=2$, $\mathrm{pH}=6$, and $\mathrm{pH}=11$ are $\left|Z_1\right|,\left|Z_2\right|$, and $\left|Z_3\right|$, respectively, then what is $\left|Z_1\right|+\left|Z_2\right|+\left|Z_3\right|$?

A tetrapeptide has $$-$$COOH group on alanine. This produces glycine (Gly), valine (Val), phenyl alanine (Phe) and alanine (Ala), on complete hydrolysis. For this tetrapeptide, the number of possible sequences (primary structures) with $$-$$NH2 group attached to a chiral center is _______.
The substituents R1 and R2 for nine peptides are listed in the table given below. How many of these peptides are positively charged at pH = 7.0 ?

| Peptide | $${R_1}$$ | $${R_2}$$ |
|---|---|---|
| I | H | H |
| II | H | $$C{H_3}$$ |
| III | $$C{H_2}COOH$$ | H |
| IV | $$C{H_2}CON{H_2}$$ | $${(C{H_2})_4}N{H_2}$$ |
| V | $$C{H_2}CON{H_2}$$ | $$C{H_2}CON{H_2}$$ |
| VI | $${(C{H_2})_4}N{H_2}$$ | $${(C{H_2})_4}N{H_2}$$ |
| VII | $$C{H_2}COOH$$ | $$C{H_2}CON{H_2}$$ |
| VIII | $$C{H_2}OH$$ | $${(C{H_2})_4}N{H_2}$$ |
| IX | $${(C{H_2})_4}N{H_2}$$ | $$C{H_3}$$ |
A decapeptide (mol. wt. 796) on complete hydrolysis gives glycine (mol. wt. 75), alanine and phenylalanine. Glycine contributes 47.0% to the total weight of the hydrolysed products. The number of glycine units present in the decapeptide is _________.
The total number of basic groups in the following form of lysine is

MCQ (Single Correct Answer)
The following carbohydrate is

The correct statement about the following disaccharide is :

Cellulose upon acetylation with excess acetic anhydride/H$$_2$$SO$$_4$$ (catalytic) gives cellulose triacetate whose structure is :
MCQ (More than One Correct Answer)

The compound(s), which on reaction with HNO3 will give the product having degree of rotation, [$$\alpha$$]D = $$-$$52.7$$^\circ$$ is (are)
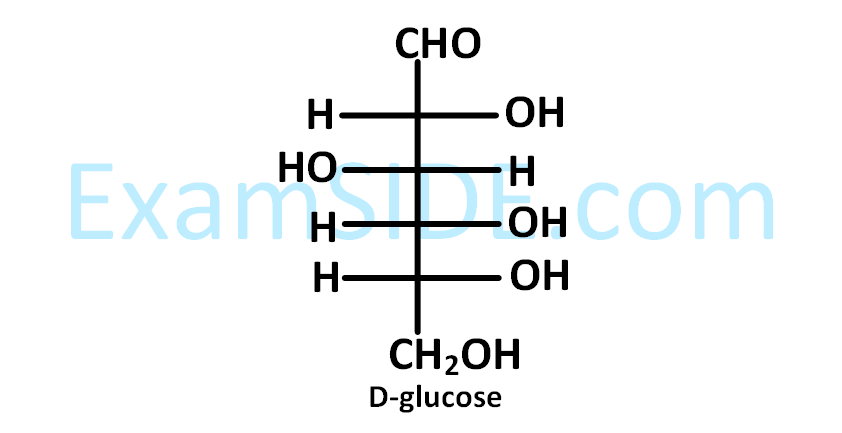
The correct structure(s) of $$\beta $$-$$L$$-glucopyranose is (are) :
For "invert sugar", the correct statement(s) is(are)
(Given : specific rotations of (+)-sucrose, (+)-maltose, L-($$-$$)-glucose and L-(+)-fructose in aqueous solution are +66$$^\circ$$, +140$$^\circ$$, $$-$$52$$^\circ$$ and +92$$^\circ$$, respectively.)
The structure of D-(+)-glucose is

The structure of L-($$-$$)-glucose is
The correct statement(s) about the following sugar X and Y is(are)

