1
JEE Advanced 2018 Paper 2 Offline
Numerical
+3
-0

To measure the quantity of $$MnC{l_2}$$ dissolved in an aqueous solution, it was completely converted to $$KMn{O_4}$$ using the reaction,
$$MnC{l_2} + {K_2}{S_2}{O_8} + {H_2}O \to KMn{O_4} + {H_2}S{O_4} + HCl$$ (equation not balanced).
Few drops of concentrated $$HCl$$ were added to this solution and gently warmed. Further, oxalic acid ($$225$$ $$mg$$) was added in portions till the colour of the permanganate ion disappeared. The quantity of $$MnC{l_2}$$ (in mg) present in the initial solution is ____________.
(Atomic weights in $$g\,\,mo{l^{ - 1}}:Mn = 55,Cl = 35.5$$ )
$$MnC{l_2} + {K_2}{S_2}{O_8} + {H_2}O \to KMn{O_4} + {H_2}S{O_4} + HCl$$ (equation not balanced).
Few drops of concentrated $$HCl$$ were added to this solution and gently warmed. Further, oxalic acid ($$225$$ $$mg$$) was added in portions till the colour of the permanganate ion disappeared. The quantity of $$MnC{l_2}$$ (in mg) present in the initial solution is ____________.
(Atomic weights in $$g\,\,mo{l^{ - 1}}:Mn = 55,Cl = 35.5$$ )
Your input ____
2
JEE Advanced 2018 Paper 2 Offline
MCQ (More than One Correct Answer)
+4
-1

Aniline reacts with mixed acid (conc. $$HN{O_3}$$ and conc. $${H_2}S{O_4}$$) at $$288$$ $$K$$ to give P $$\left( {51\% } \right),$$ Q $$\left( {47\% } \right)$$ and R $$\left( {2\% } \right).$$ The major product(s) of the following reaction sequence is (are)
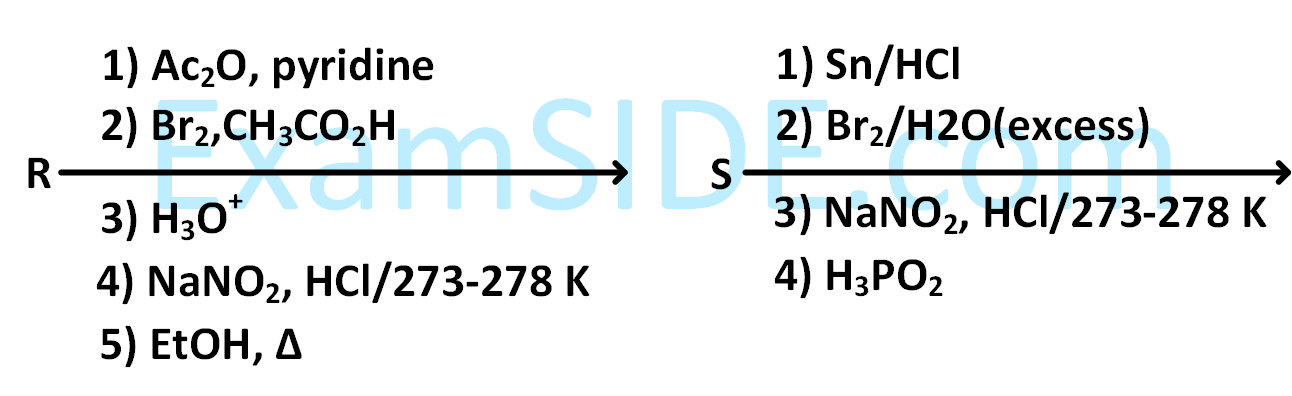

3
JEE Advanced 2018 Paper 2 Offline
Numerical
+3
-0

The surface of copper gets tarnished by the formation of copper oxide. $${N_2}$$ gas was passed to prevent the oxide formation during heating of copper at $$1250$$ $$K.$$ However, the $${N_2}$$ gas contains $$1$$ mole % of water vapor as impurity. The water vapor oxidises copper as per the reaction given below : $$2Cu\left( s \right) + {H_2}O\left( g \right) \to C{u_2}O\left( s \right) + {H_2}\left( g \right)$$
$${P_{H2}}$$ is the minimum partial pressure of $${H_2}$$ (in bar) needed to prevent the oxidation at $$1250$$ $$K.$$ The value of $$\ln \left( {{P_{H2}}} \right)$$ is ________.
Given: total pressure $$=1$$ bar, $$R$$ (universal gas constant ) $$=$$ $$8J{K^{ - 1}}\,\,mo{l^{ - 1}},$$ $$\ln \left( {10} \right) = 2.3.\,$$ $$Cu(s)$$ and $$C{u_2}O\left( s \right)$$ are naturally immiscible.
At $$1250$$ $$K:2Cu(s)$$ $$ + {\raise0.5ex\hbox{$\scriptstyle 1$} \kern-0.1em/\kern-0.15em \lower0.25ex\hbox{$\scriptstyle 2$}}{O_2}\left( g \right) \to C{u_2}O\left( s \right);$$ $$\Delta {G^ \circ } = - 78,000J\,mo{l^{ - 1}}$$
$${H_2}\left( g \right) + {\raise0.5ex\hbox{$\scriptstyle 1$} \kern-0.1em/\kern-0.15em \lower0.25ex\hbox{$\scriptstyle 2$}}{O_2}\left( g \right) \to {H_2}O\left( g \right);$$
$$\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,$$ $$\Delta {G^ \circ } = - 1,78,000J\,mo{l^{ - 1}};$$ ($$G$$ is the Gibbs energy)
$${P_{H2}}$$ is the minimum partial pressure of $${H_2}$$ (in bar) needed to prevent the oxidation at $$1250$$ $$K.$$ The value of $$\ln \left( {{P_{H2}}} \right)$$ is ________.
Given: total pressure $$=1$$ bar, $$R$$ (universal gas constant ) $$=$$ $$8J{K^{ - 1}}\,\,mo{l^{ - 1}},$$ $$\ln \left( {10} \right) = 2.3.\,$$ $$Cu(s)$$ and $$C{u_2}O\left( s \right)$$ are naturally immiscible.
At $$1250$$ $$K:2Cu(s)$$ $$ + {\raise0.5ex\hbox{$\scriptstyle 1$} \kern-0.1em/\kern-0.15em \lower0.25ex\hbox{$\scriptstyle 2$}}{O_2}\left( g \right) \to C{u_2}O\left( s \right);$$ $$\Delta {G^ \circ } = - 78,000J\,mo{l^{ - 1}}$$
$${H_2}\left( g \right) + {\raise0.5ex\hbox{$\scriptstyle 1$} \kern-0.1em/\kern-0.15em \lower0.25ex\hbox{$\scriptstyle 2$}}{O_2}\left( g \right) \to {H_2}O\left( g \right);$$
$$\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,$$ $$\Delta {G^ \circ } = - 1,78,000J\,mo{l^{ - 1}};$$ ($$G$$ is the Gibbs energy)
Your input ____
4
JEE Advanced 2018 Paper 2 Offline
Numerical
+3
-0

For the given compound $$X,$$ the total number of optically active stereoisomers is ____________.
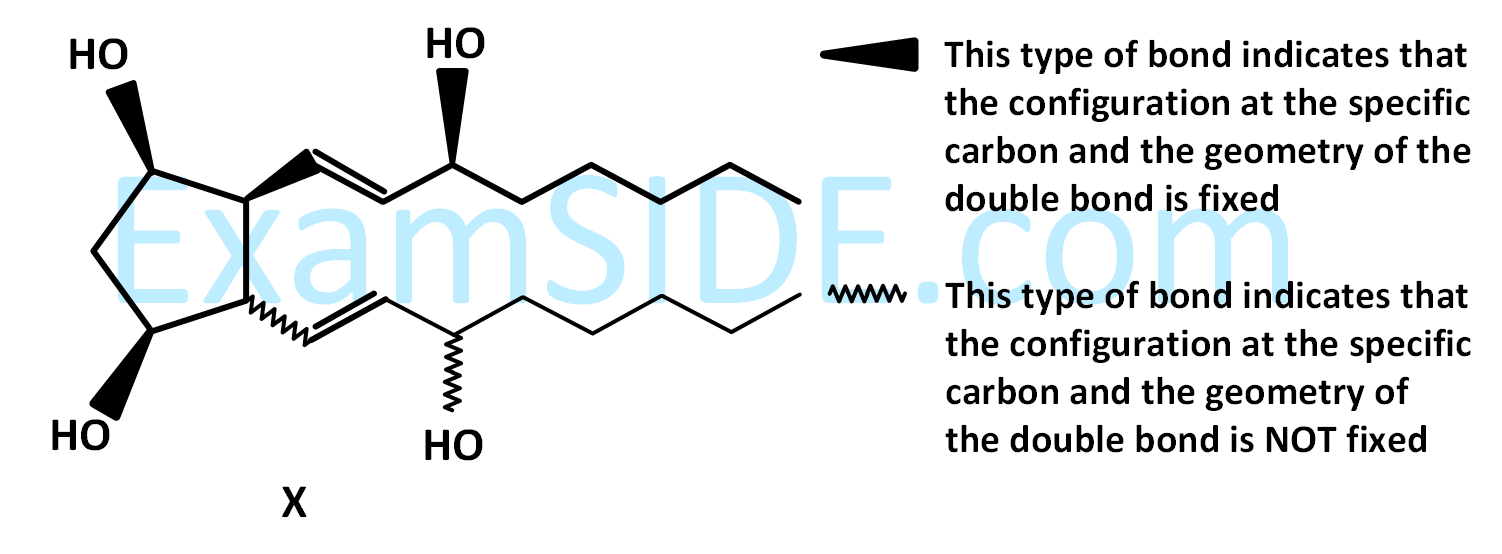



Your input ____
Paper analysis
Total Questions
Chemistry
18
Mathematics
18
Physics
18
More papers of JEE Advanced
JEE Advanced 2025 Paper 2 Online
JEE Advanced 2025 Paper 1 Online
JEE Advanced 2024 Paper 2 Online
JEE Advanced 2024 Paper 1 Online
JEE Advanced 2023 Paper 2 Online
JEE Advanced 2023 Paper 1 Online
JEE Advanced 2022 Paper 2 Online
JEE Advanced 2022 Paper 1 Online
JEE Advanced 2021 Paper 2 Online
JEE Advanced 2021 Paper 1 Online
JEE Advanced 2020 Paper 2 Offline
JEE Advanced 2020 Paper 1 Offline
JEE Advanced 2019 Paper 2 Offline
JEE Advanced 2019 Paper 1 Offline
JEE Advanced 2018 Paper 2 Offline
JEE Advanced 2018 Paper 1 Offline
JEE Advanced 2017 Paper 2 Offline
JEE Advanced 2017 Paper 1 Offline
JEE Advanced 2016 Paper 2 Offline
JEE Advanced 2016 Paper 1 Offline
JEE Advanced 2015 Paper 2 Offline
JEE Advanced 2015 Paper 1 Offline
JEE Advanced 2014 Paper 2 Offline
JEE Advanced 2014 Paper 1 Offline
JEE Advanced 2013 Paper 2 Offline
JEE Advanced 2013 Paper 1 Offline
IIT-JEE 2012 Paper 2 Offline
IIT-JEE 2012 Paper 1 Offline
IIT-JEE 2011 Paper 1 Offline
IIT-JEE 2011 Paper 2 Offline
IIT-JEE 2010 Paper 1 Offline
IIT-JEE 2010 Paper 2 Offline
IIT-JEE 2009 Paper 2 Offline
IIT-JEE 2009 Paper 1 Offline
IIT-JEE 2008 Paper 2 Offline
IIT-JEE 2008 Paper 1 Offline
IIT-JEE 2007
IIT-JEE 2007 Paper 2 Offline
IIT-JEE 2006
IIT-JEE 2006 Screening
IIT-JEE 2005 Screening
IIT-JEE 2005
IIT-JEE 2004 Screening
IIT-JEE 2004
IIT-JEE 2003
IIT-JEE 2003 Screening
IIT-JEE 2002
IIT-JEE 2002 Screening
IIT-JEE 2001 Screening
IIT-JEE 2001
IIT-JEE 2000 Screening
IIT-JEE 2000
IIT-JEE 1999 Screening
IIT-JEE 1999
IIT-JEE 1998 Screening
IIT-JEE 1998
IIT-JEE 1997
IIT-JEE 1996
IIT-JEE 1995 Screening
IIT-JEE 1995
IIT-JEE 1994
IIT-JEE 1993
IIT-JEE 1992
IIT-JEE 1991
IIT-JEE 1990
IIT-JEE 1989
IIT-JEE 1988
IIT-JEE 1987
IIT-JEE 1986
IIT-JEE 1985
IIT-JEE 1984
IIT-JEE 1983
IIT-JEE 1982
IIT-JEE 1981
IIT-JEE 1980
IIT-JEE 1979
IIT-JEE 1978
JEE Advanced
Papers
2020
2019
2018
2017
2016
1997
1996
1994
1993
1992
1991
1990
1989
1988
1987
1986
1985
1984
1983
1982
1981
1980
1979
1978



