1
JEE Advanced 2018 Paper 2 Offline
MCQ (Single Correct Answer)
+3
-0.75

The electric field $$E$$ is measured at a point $$P(0,0,d)$$ generated due to various charge distributions and the dependence of $$E$$ on $$d$$ is found to be different for different charge distributions. List-$${\rm I}$$ contains different relations between $$E$$ and $$d$$. List-$${\rm II}$$ describes different electric charge distributions, along with their locations. Match the functions in List-$${\rm I}$$ with the related charge distributions in List-$${\rm II}$$.
| LIST - I | LIST - II | ||
|---|---|---|---|
| P. | $$E$$ is independent of $$d$$ | 1. | A point charge Q at the origin |
| Q. | $$E\, \propto \,1/d$$ | 2. | A small dipole with point charges $$Q$$ at $$\left( {0,0,l} \right)$$ and $$-Q$$ at $$\left( {0,0, - l} \right).$$ Take $$2l < < d$$ |
| R. | $$E\, \propto \,1/{d^2}$$ | 3. | An infinite line charge coincident with the x-axis, with uniform linear charge density $$\lambda $$ |
| S. | $$E\, \propto \,1/{d^3}$$ | 4. | Two infinite wires carrying uniform linear charge density parallel to the $$x$$-axis. The one along $$\left( {y = 0,z = l} \right)$$ has a charge density $$ + \lambda $$ and the one along $$\left( {y = 0,z = - l} \right)$$ has a charge density Take |
| 5. | Infinite plane charge coincident
with the $$xy$$-plane with uniform surface charge density |
||
2
JEE Advanced 2018 Paper 2 Offline
MCQ (Single Correct Answer)
+3
-1

One mole of a monatomic ideal gas undergoes four thermodynamic processes as shown schematically in the $$PV$$-diagram below. Among these four processes, one is isobaric, one is isochoric, one is isothermal and one is adiabatic. Match the processes mentioned in List-I with the corresponding statements in List-II.
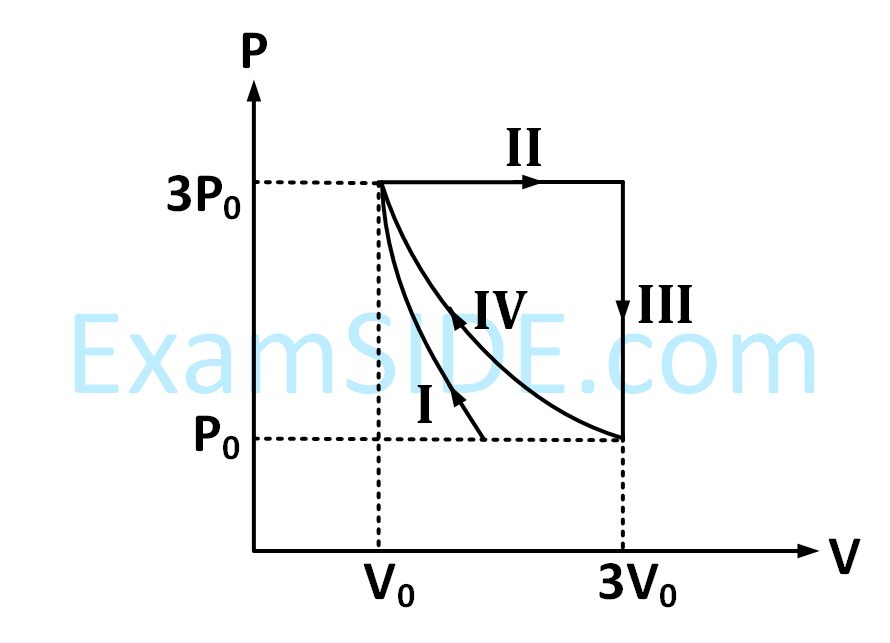

| LIST - I | LIST - II | ||
|---|---|---|---|
| P. | In process I | 1. | Work done by the gas is zero |
| Q. | In process II | 2. | Temperature of the gas remains unchanged |
| R. | In process III | 3. | No heat is exchanged between the gas and its surroundings |
| S. | In process IV | 4. | Work done by the gas is 6P0V0 |
3
JEE Advanced 2018 Paper 2 Offline
MCQ (Single Correct Answer)
+3
-0.75

In the List-$${\rm I}$$ below, four different paths of a particle are given as functions of time. In these functions, $$\alpha $$ and $$\beta $$ are positive constants of appropriate dimensions and $$\alpha \ne \beta $$ In each case, the force acting on the particle is either zero or conservative. In List-$${\rm I}{\rm I}$$, five physical quantities of the particle are mentioned $$\overrightarrow p $$ is the linear momentum, $$\overrightarrow L $$ is the angular momentum about the origin, $$K$$ is the kinetic energy, $$U$$ is the potential energy and $$E$$ is the total energy. Match each path in List-$${\rm I}$$ with those quantities in List-$${\rm II}$$, which are conserved for that path.
| LIST - I | LIST - II | ||
|---|---|---|---|
| P. | $$\overrightarrow r $$(t)=$$\alpha $$ $$t\,\widehat i + \beta t\widehat j$$ | 1. | $$\overrightarrow p $$ |
| Q. | $$\overrightarrow r \left( t \right) = \alpha \cos \,\omega t\,\widehat i + \beta \sin \omega t\,\widehat j$$ | 2. | $$\overrightarrow L $$ |
| R. | $$\overrightarrow r \left( t \right) = \alpha \left( {\cos \omega t\,\widehat i + \sin \omega t\widehat j} \right)$$ | 3. | K |
| S. | $$\overrightarrow r \left( t \right) = \alpha t\,\widehat i + {\beta \over 2}{t^2}\widehat j$$ | 4. | U |
| 5. | E | ||
4
JEE Advanced 2018 Paper 2 Offline
MCQ (More than One Correct Answer)
+4
-1

A particle of mass $$m$$ is initially at rest at the origin. It is subjected to a force and starts moving along the $$x$$-axis. Its kinetic energy $$K$$ changes with time as $$dK/dt = \gamma t,$$ where $$\gamma $$ is a positive constant of appropriate dimensions. Which of a positive constant of appropriate dimensions. Which of the following statement is (are) true?
Paper analysis
Total Questions
Chemistry
18
Mathematics
18
Physics
18
More papers of JEE Advanced
JEE Advanced 2025 Paper 2 Online
JEE Advanced 2025 Paper 1 Online
JEE Advanced 2024 Paper 2 Online
JEE Advanced 2024 Paper 1 Online
JEE Advanced 2023 Paper 2 Online
JEE Advanced 2023 Paper 1 Online
JEE Advanced 2022 Paper 2 Online
JEE Advanced 2022 Paper 1 Online
JEE Advanced 2021 Paper 2 Online
JEE Advanced 2021 Paper 1 Online
JEE Advanced 2020 Paper 2 Offline
JEE Advanced 2020 Paper 1 Offline
JEE Advanced 2019 Paper 2 Offline
JEE Advanced 2019 Paper 1 Offline
JEE Advanced 2018 Paper 2 Offline
JEE Advanced 2018 Paper 1 Offline
JEE Advanced 2017 Paper 2 Offline
JEE Advanced 2017 Paper 1 Offline
JEE Advanced 2016 Paper 2 Offline
JEE Advanced 2016 Paper 1 Offline
JEE Advanced 2015 Paper 2 Offline
JEE Advanced 2015 Paper 1 Offline
JEE Advanced 2014 Paper 2 Offline
JEE Advanced 2014 Paper 1 Offline
JEE Advanced 2013 Paper 2 Offline
JEE Advanced 2013 Paper 1 Offline
IIT-JEE 2012 Paper 2 Offline
IIT-JEE 2012 Paper 1 Offline
IIT-JEE 2011 Paper 1 Offline
IIT-JEE 2011 Paper 2 Offline
IIT-JEE 2010 Paper 2 Offline
IIT-JEE 2010 Paper 1 Offline
IIT-JEE 2009 Paper 2 Offline
IIT-JEE 2009 Paper 1 Offline
IIT-JEE 2008 Paper 2 Offline
IIT-JEE 2008 Paper 1 Offline
IIT-JEE 2007
IIT-JEE 2007 Paper 2 Offline
IIT-JEE 2006
IIT-JEE 2006 Screening
IIT-JEE 2005 Screening
IIT-JEE 2005
IIT-JEE 2004
IIT-JEE 2004 Screening
IIT-JEE 2003
IIT-JEE 2003 Screening
IIT-JEE 2002
IIT-JEE 2002 Screening
IIT-JEE 2001
IIT-JEE 2001 Screening
IIT-JEE 2000 Screening
IIT-JEE 2000
IIT-JEE 1999 Screening
IIT-JEE 1999
IIT-JEE 1998
IIT-JEE 1998 Screening
IIT-JEE 1997
IIT-JEE 1996
IIT-JEE 1995 Screening
IIT-JEE 1995
IIT-JEE 1994
IIT-JEE 1993
IIT-JEE 1992
IIT-JEE 1991
IIT-JEE 1990
IIT-JEE 1989
IIT-JEE 1988
IIT-JEE 1987
IIT-JEE 1986
IIT-JEE 1985
IIT-JEE 1984
IIT-JEE 1983
IIT-JEE 1982
IIT-JEE 1981
IIT-JEE 1980
IIT-JEE 1979
IIT-JEE 1978
JEE Advanced
Papers
2020
2019
2018
2017
2016
1997
1996
1994
1993
1992
1991
1990
1989
1988
1987
1986
1985
1984
1983
1982
1981
1980
1979
1978