1
JEE Advanced 2018 Paper 2 Offline
MCQ (More than One Correct Answer)
+4
-2

The Fischer presentation of $$D$$-glucose is given below.
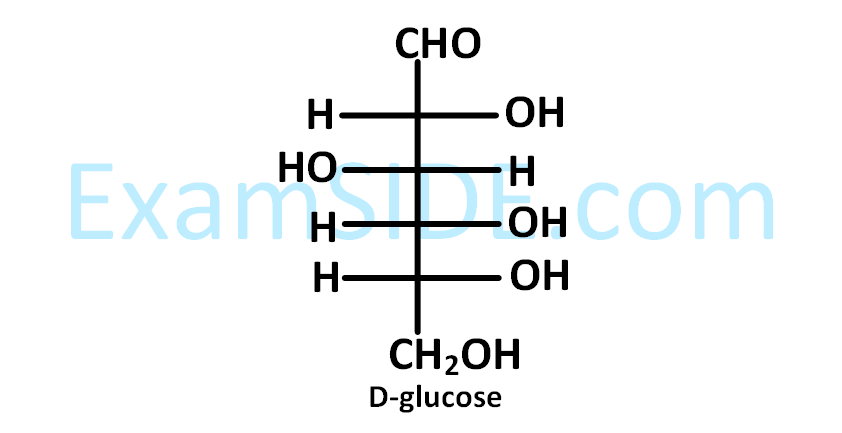
The correct structure(s) of $$\beta $$-$$L$$-glucopyranose is (are) :

The correct structure(s) of $$\beta $$-$$L$$-glucopyranose is (are) :
2
JEE Advanced 2018 Paper 2 Offline
MCQ (More than One Correct Answer)
+4
-1

The correct option(s) to distinguish nitrate salts of $$M{n^{2 + }}$$ and $$C{u^{2 + }}$$ taken separately is (are)
3
JEE Advanced 2018 Paper 2 Offline
Numerical
+3
-0

The total number of compounds having at least one bridging oxo group among the molecules given below is _________.
$${N_2}{O_3},{N_2}{O_5},$$ $${P_4}{O_6},{P_4}{O_7},$$ $${H_4}{P_2}{O_5},{H_5}{P_3}{O_{10}},$$ $${H_2}{S_2}{O_3},{H_2}{S_2}{O_5}$$
$${N_2}{O_3},{N_2}{O_5},$$ $${P_4}{O_6},{P_4}{O_7},$$ $${H_4}{P_2}{O_5},{H_5}{P_3}{O_{10}},$$ $${H_2}{S_2}{O_3},{H_2}{S_2}{O_5}$$
Your input ____
4
JEE Advanced 2018 Paper 2 Offline
MCQ (More than One Correct Answer)
+4
-1

The correct option(s) regarding the complex
$${\left[ {Co\left( {en} \right){{\left( {N{H_3}} \right)}_3}\left( {{H_2}O} \right)} \right]^{3 + }}\,\,$$ $$\left( {en = {H_2}NC{H_2}C{H_2}N{H_2}} \right)$$ is (are)
$${\left[ {Co\left( {en} \right){{\left( {N{H_3}} \right)}_3}\left( {{H_2}O} \right)} \right]^{3 + }}\,\,$$ $$\left( {en = {H_2}NC{H_2}C{H_2}N{H_2}} \right)$$ is (are)
Paper analysis
Total Questions
Chemistry
18
Mathematics
18
Physics
18
More papers of JEE Advanced
JEE Advanced 2025 Paper 2 Online
JEE Advanced 2025 Paper 1 Online
JEE Advanced 2024 Paper 2 Online
JEE Advanced 2024 Paper 1 Online
JEE Advanced 2023 Paper 2 Online
JEE Advanced 2023 Paper 1 Online
JEE Advanced 2022 Paper 2 Online
JEE Advanced 2022 Paper 1 Online
JEE Advanced 2021 Paper 2 Online
JEE Advanced 2021 Paper 1 Online
JEE Advanced 2020 Paper 2 Offline
JEE Advanced 2020 Paper 1 Offline
JEE Advanced 2019 Paper 2 Offline
JEE Advanced 2019 Paper 1 Offline
JEE Advanced 2018 Paper 2 Offline
JEE Advanced 2018 Paper 1 Offline
JEE Advanced 2017 Paper 2 Offline
JEE Advanced 2017 Paper 1 Offline
JEE Advanced 2016 Paper 2 Offline
JEE Advanced 2016 Paper 1 Offline
JEE Advanced 2015 Paper 2 Offline
JEE Advanced 2015 Paper 1 Offline
JEE Advanced 2014 Paper 2 Offline
JEE Advanced 2014 Paper 1 Offline
JEE Advanced 2013 Paper 2 Offline
JEE Advanced 2013 Paper 1 Offline
IIT-JEE 2012 Paper 2 Offline
IIT-JEE 2012 Paper 1 Offline
IIT-JEE 2011 Paper 1 Offline
IIT-JEE 2011 Paper 2 Offline
IIT-JEE 2010 Paper 1 Offline
IIT-JEE 2010 Paper 2 Offline
IIT-JEE 2009 Paper 2 Offline
IIT-JEE 2009 Paper 1 Offline
IIT-JEE 2008 Paper 2 Offline
IIT-JEE 2008 Paper 1 Offline
IIT-JEE 2007
IIT-JEE 2007 Paper 2 Offline
IIT-JEE 2006
IIT-JEE 2006 Screening
IIT-JEE 2005 Screening
IIT-JEE 2005
IIT-JEE 2004 Screening
IIT-JEE 2004
IIT-JEE 2003
IIT-JEE 2003 Screening
IIT-JEE 2002
IIT-JEE 2002 Screening
IIT-JEE 2001 Screening
IIT-JEE 2001
IIT-JEE 2000 Screening
IIT-JEE 2000
IIT-JEE 1999 Screening
IIT-JEE 1999
IIT-JEE 1998 Screening
IIT-JEE 1998
IIT-JEE 1997
IIT-JEE 1996
IIT-JEE 1995 Screening
IIT-JEE 1995
IIT-JEE 1994
IIT-JEE 1993
IIT-JEE 1992
IIT-JEE 1991
IIT-JEE 1990
IIT-JEE 1989
IIT-JEE 1988
IIT-JEE 1987
IIT-JEE 1986
IIT-JEE 1985
IIT-JEE 1984
IIT-JEE 1983
IIT-JEE 1982
IIT-JEE 1981
IIT-JEE 1980
IIT-JEE 1979
IIT-JEE 1978
JEE Advanced
Papers
2020
2019
2018
2017
2016
1997
1996
1994
1993
1992
1991
1990
1989
1988
1987
1986
1985
1984
1983
1982
1981
1980
1979
1978



