1
JEE Advanced 2018 Paper 2 Offline
MCQ (More than One Correct Answer)
+4
-1

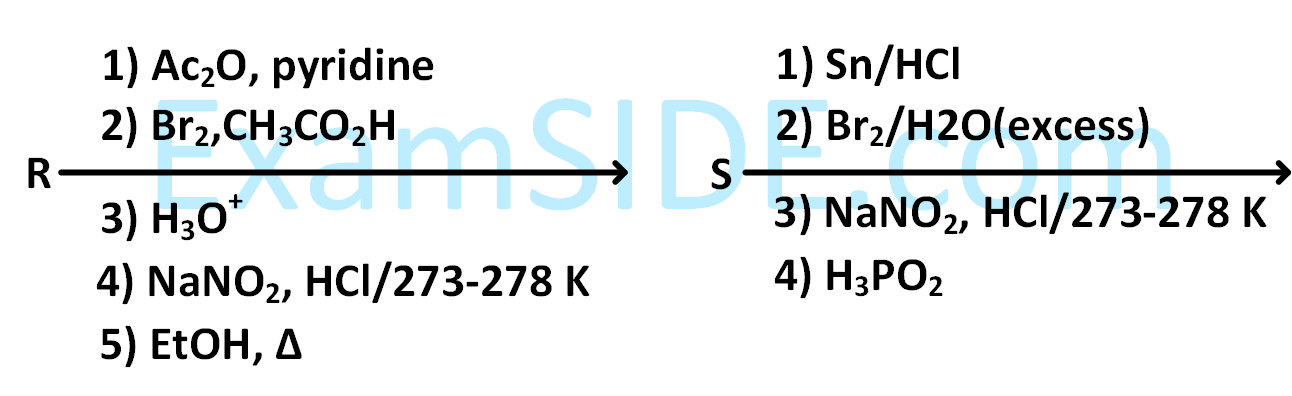
Aniline reacts with mixed acid (conc. $$HN{O_3}$$ and conc. $${H_2}S{O_4}$$) at $$288$$ $$K$$ to give P $$\left( {51\% } \right),$$ Q $$\left( {47\% } \right)$$ and R $$\left( {2\% } \right).$$ The major product(s) of the following reaction sequence is (are)

2
JEE Advanced 2018 Paper 2 Offline
MCQ (More than One Correct Answer)
+4
-1

For a first order reaction $$A\left( g \right) \to 2B\left( g \right) + C\left( g \right)$$ at constant volume and $$300K,$$ the total pressure at the beginning $$(t=0)$$ and at time $$t$$ are $${P_0}$$ and $${P_1},$$ respectively. Initially, only $$A$$ is present with concentration $${\left[ A \right]_0},$$ and $${t_{1/3}}$$ is the time required for the partial pressure of $$A$$ to reach $$1/{3^{rd}}$$ of its initial value. The correct option(s) is (are) (Assume that all these gases behave as ideal gases)
3
JEE Advanced 2018 Paper 2 Offline
MCQ (Single Correct Answer)
+3
-0.75

Match each set of hybrid orbitals from LIST - A with complex(es) given in LIST - B
The correct option is
| List - A | List - B | |||
|---|---|---|---|---|
| P. | dsp2 | 1. | [FeF6]4- | |
| Q. | sp3 | 2. | [Ti(H2O)3Cl3] | |
| R. | sp3d2 | 3. | [Cr(NH3)6]3+ | |
| S. | d2sp3 | 4. | [FeCl4]2- | |
| 5. | Ni(CO)4 | |||
| 6. | [Ni(CN)4]2- |
The correct option is
4
JEE Advanced 2018 Paper 2 Offline
Numerical
+3
-0

Consider the following reversible reaction, $$A\left( g \right) + B\left( g \right) \to AB\left( g \right).$$
The activation energy of the backward reaction exceeds that of the forward reaction by $$2RT$$ (in $$J\,mo{l^{ - 1}}$$). If the pre-exponential factor of the forward reaction is $$4$$ times that of the reverse reaction, the absolute value of $$\Delta {G^ \circ }$$ (in $$J\,mo{l^{ - 1}}$$ ) for the reaction at $$300$$ $$K$$ is ____________.
(Given; $$\ln \left( 2 \right) = 0.7,RT = 2500$$ $$J\,mo{l^{ - 1}}$$ at $$300$$ $$K$$ and $$G$$ is the Gibbs energy)
The activation energy of the backward reaction exceeds that of the forward reaction by $$2RT$$ (in $$J\,mo{l^{ - 1}}$$). If the pre-exponential factor of the forward reaction is $$4$$ times that of the reverse reaction, the absolute value of $$\Delta {G^ \circ }$$ (in $$J\,mo{l^{ - 1}}$$ ) for the reaction at $$300$$ $$K$$ is ____________.
(Given; $$\ln \left( 2 \right) = 0.7,RT = 2500$$ $$J\,mo{l^{ - 1}}$$ at $$300$$ $$K$$ and $$G$$ is the Gibbs energy)
Your input ____
Paper analysis
Total Questions
Chemistry
18
Mathematics
18
Physics
18
More papers of JEE Advanced
JEE Advanced 2025 Paper 2 Online
JEE Advanced 2025 Paper 1 Online
JEE Advanced 2024 Paper 2 Online
JEE Advanced 2024 Paper 1 Online
JEE Advanced 2023 Paper 2 Online
JEE Advanced 2023 Paper 1 Online
JEE Advanced 2022 Paper 2 Online
JEE Advanced 2022 Paper 1 Online
JEE Advanced 2021 Paper 2 Online
JEE Advanced 2021 Paper 1 Online
JEE Advanced 2020 Paper 2 Offline
JEE Advanced 2020 Paper 1 Offline
JEE Advanced 2019 Paper 2 Offline
JEE Advanced 2019 Paper 1 Offline
JEE Advanced 2018 Paper 2 Offline
JEE Advanced 2018 Paper 1 Offline
JEE Advanced 2017 Paper 2 Offline
JEE Advanced 2017 Paper 1 Offline
JEE Advanced 2016 Paper 2 Offline
JEE Advanced 2016 Paper 1 Offline
JEE Advanced 2015 Paper 2 Offline
JEE Advanced 2015 Paper 1 Offline
JEE Advanced 2014 Paper 2 Offline
JEE Advanced 2014 Paper 1 Offline
JEE Advanced 2013 Paper 2 Offline
JEE Advanced 2013 Paper 1 Offline
IIT-JEE 2012 Paper 2 Offline
IIT-JEE 2012 Paper 1 Offline
IIT-JEE 2011 Paper 1 Offline
IIT-JEE 2011 Paper 2 Offline
IIT-JEE 2010 Paper 1 Offline
IIT-JEE 2010 Paper 2 Offline
IIT-JEE 2009 Paper 2 Offline
IIT-JEE 2009 Paper 1 Offline
IIT-JEE 2008 Paper 2 Offline
IIT-JEE 2008 Paper 1 Offline
IIT-JEE 2007
IIT-JEE 2007 Paper 2 Offline
IIT-JEE 2006
IIT-JEE 2006 Screening
IIT-JEE 2005 Screening
IIT-JEE 2005
IIT-JEE 2004 Screening
IIT-JEE 2004
IIT-JEE 2003
IIT-JEE 2003 Screening
IIT-JEE 2002
IIT-JEE 2002 Screening
IIT-JEE 2001 Screening
IIT-JEE 2001
IIT-JEE 2000 Screening
IIT-JEE 2000
IIT-JEE 1999 Screening
IIT-JEE 1999
IIT-JEE 1998 Screening
IIT-JEE 1998
IIT-JEE 1997
IIT-JEE 1996
IIT-JEE 1995 Screening
IIT-JEE 1995
IIT-JEE 1994
IIT-JEE 1993
IIT-JEE 1992
IIT-JEE 1991
IIT-JEE 1990
IIT-JEE 1989
IIT-JEE 1988
IIT-JEE 1987
IIT-JEE 1986
IIT-JEE 1985
IIT-JEE 1984
IIT-JEE 1983
IIT-JEE 1982
IIT-JEE 1981
IIT-JEE 1980
IIT-JEE 1979
IIT-JEE 1978
JEE Advanced
Papers
2020
2019
2018
2017
2016
1997
1996
1994
1993
1992
1991
1990
1989
1988
1987
1986
1985
1984
1983
1982
1981
1980
1979
1978







