1
JEE Advanced 2018 Paper 2 Offline
MCQ (Single Correct Answer)
+3
-0.75

Match each set of hybrid orbitals from LIST - A with complex(es) given in LIST - B
The correct option is
| List - A | List - B | |||
|---|---|---|---|---|
| P. | dsp2 | 1. | [FeF6]4- | |
| Q. | sp3 | 2. | [Ti(H2O)3Cl3] | |
| R. | sp3d2 | 3. | [Cr(NH3)6]3+ | |
| S. | d2sp3 | 4. | [FeCl4]2- | |
| 5. | Ni(CO)4 | |||
| 6. | [Ni(CN)4]2- |
The correct option is
2
JEE Advanced 2018 Paper 2 Offline
MCQ (Single Correct Answer)
+3
-1

Dilution processes of different aqueous solutions, with water, are given in LIST - I. The effects of dilution of the solutions on $$\left[ {{H^ + }} \right]$$ are given in LIST - II
(Note: Degree of dissociation (a) of weak acid and weak base is $$<<1;$$ degree of hydrolysis of salt $$<<1;$$ $$\left[ {{H^ + }} \right]$$ represents the concentration of $${H^ + }$$ ions)
Match each process given in LIST-I with one or more effect(s) in LIST-II. The correct option is :
(Note: Degree of dissociation (a) of weak acid and weak base is $$<<1;$$ degree of hydrolysis of salt $$<<1;$$ $$\left[ {{H^ + }} \right]$$ represents the concentration of $${H^ + }$$ ions)
| LIST-I | LIST-II | ||
|---|---|---|---|
| P. | (10 mL of 0.1 M NaOH + 20 mL of 0.1 M acetic acid) diluted to 60 mL |
1. | the value of [H+] does not change on dilution |
| Q. | (20 mL of 0.1 M NaOH + 20 mL of 0.1 M acetic acid) diluted to 80 mL |
2. | the value of [H+] changes to half of its initial value on dilution |
| R. | (20 mL of 0.1 M HCL + 20 mL of 0.1 M ammonia solution) diluted to 80 mL |
3. | the value of [H+] changes to two times of its initial value on dilution |
| S. | 10 mL saturated solution of Ni(OH)2 in equilibrium with excess solid Ni(OH)2 is diluted to 20 mL (solid Ni(OH)2 is still present after dilution). |
4. | the value of [H+] changes to $${1 \over {\sqrt 2 }}$$ times of its initial value on dilution |
| 5. | the value of [H+] changes to $$\sqrt 2 $$ times of its initial value on dilution |
Match each process given in LIST-I with one or more effect(s) in LIST-II. The correct option is :
3
JEE Advanced 2018 Paper 2 Offline
MCQ (Single Correct Answer)
+3
-1

The desired product $$X$$ can be prepared by reacting the major product of the reactions in LIST-I with one or more appropriate reagents in LIST-II (given, order of migratory aptitude: aryl > alkyl > hydrogen)
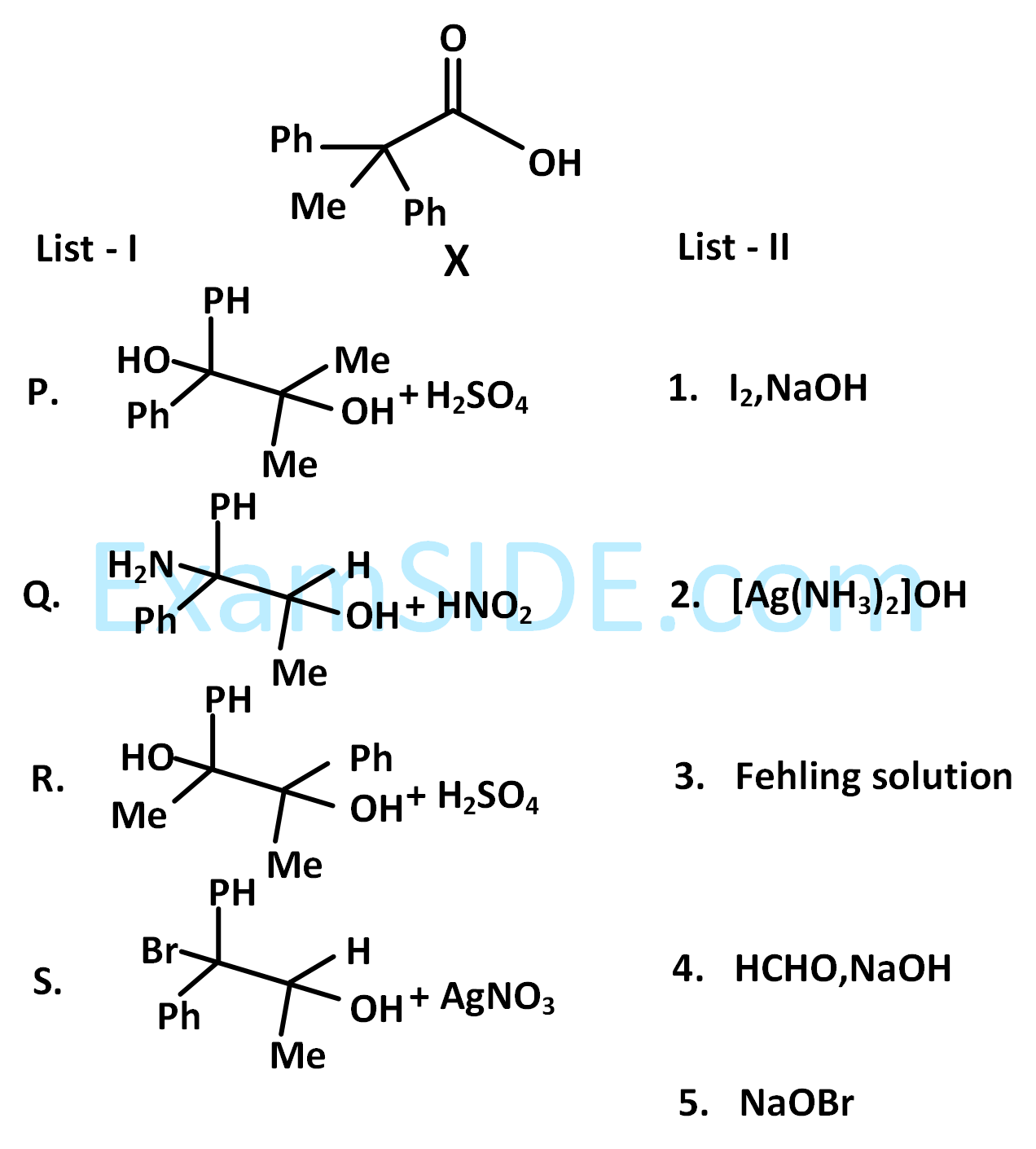
The correct option is

The correct option is
4
JEE Advanced 2018 Paper 2 Offline
MCQ (Single Correct Answer)
+3
-1

LIST-I contains reactions and LIST-II contains major products.
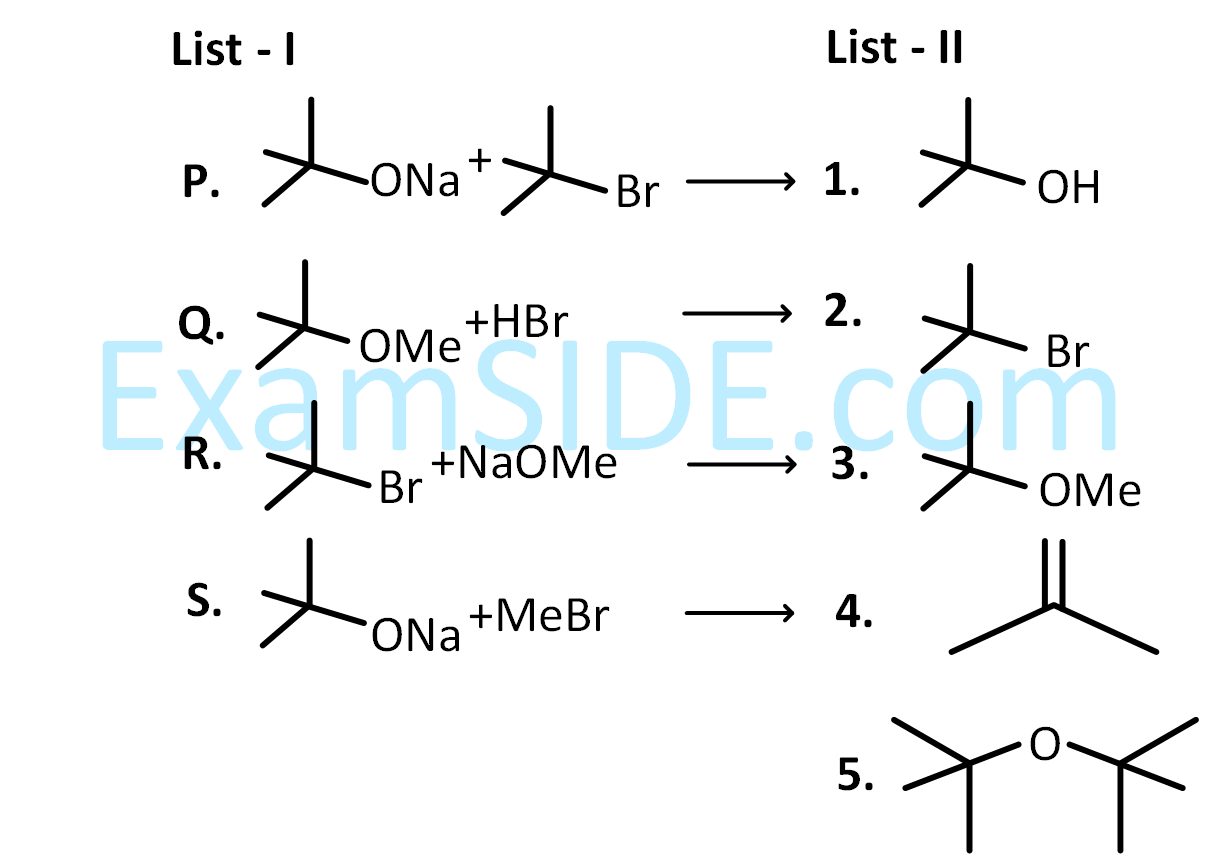
Match the reaction in LIST-I with one or more products in LIST-II and choose the correct option.

Match the reaction in LIST-I with one or more products in LIST-II and choose the correct option.
Paper analysis
Total Questions
Chemistry
18
Mathematics
18
Physics
18
More papers of JEE Advanced
JEE Advanced 2025 Paper 2 Online
JEE Advanced 2025 Paper 1 Online
JEE Advanced 2024 Paper 2 Online
JEE Advanced 2024 Paper 1 Online
JEE Advanced 2023 Paper 2 Online
JEE Advanced 2023 Paper 1 Online
JEE Advanced 2022 Paper 2 Online
JEE Advanced 2022 Paper 1 Online
JEE Advanced 2021 Paper 2 Online
JEE Advanced 2021 Paper 1 Online
JEE Advanced 2020 Paper 2 Offline
JEE Advanced 2020 Paper 1 Offline
JEE Advanced 2019 Paper 2 Offline
JEE Advanced 2019 Paper 1 Offline
JEE Advanced 2018 Paper 2 Offline
JEE Advanced 2018 Paper 1 Offline
JEE Advanced 2017 Paper 2 Offline
JEE Advanced 2017 Paper 1 Offline
JEE Advanced 2016 Paper 2 Offline
JEE Advanced 2016 Paper 1 Offline
JEE Advanced 2015 Paper 2 Offline
JEE Advanced 2015 Paper 1 Offline
JEE Advanced 2014 Paper 2 Offline
JEE Advanced 2014 Paper 1 Offline
JEE Advanced 2013 Paper 2 Offline
JEE Advanced 2013 Paper 1 Offline
IIT-JEE 2012 Paper 2 Offline
IIT-JEE 2012 Paper 1 Offline
IIT-JEE 2011 Paper 1 Offline
IIT-JEE 2011 Paper 2 Offline
IIT-JEE 2010 Paper 1 Offline
IIT-JEE 2010 Paper 2 Offline
IIT-JEE 2009 Paper 2 Offline
IIT-JEE 2009 Paper 1 Offline
IIT-JEE 2008 Paper 2 Offline
IIT-JEE 2008 Paper 1 Offline
IIT-JEE 2007
IIT-JEE 2007 Paper 2 Offline
IIT-JEE 2006
IIT-JEE 2006 Screening
IIT-JEE 2005 Screening
IIT-JEE 2005
IIT-JEE 2004 Screening
IIT-JEE 2004
IIT-JEE 2003
IIT-JEE 2003 Screening
IIT-JEE 2002
IIT-JEE 2002 Screening
IIT-JEE 2001 Screening
IIT-JEE 2001
IIT-JEE 2000 Screening
IIT-JEE 2000
IIT-JEE 1999 Screening
IIT-JEE 1999
IIT-JEE 1998 Screening
IIT-JEE 1998
IIT-JEE 1997
IIT-JEE 1996
IIT-JEE 1995 Screening
IIT-JEE 1995
IIT-JEE 1994
IIT-JEE 1993
IIT-JEE 1992
IIT-JEE 1991
IIT-JEE 1990
IIT-JEE 1989
IIT-JEE 1988
IIT-JEE 1987
IIT-JEE 1986
IIT-JEE 1985
IIT-JEE 1984
IIT-JEE 1983
IIT-JEE 1982
IIT-JEE 1981
IIT-JEE 1980
IIT-JEE 1979
IIT-JEE 1978
JEE Advanced
Papers
2020
2019
2018
2017
2016
1997
1996
1994
1993
1992
1991
1990
1989
1988
1987
1986
1985
1984
1983
1982
1981
1980
1979
1978